There are many hazards inherent in the oil and gas industry, and risk assessments are conducted to ensure that risk control measures are put in place to prevent the realization of those hazards and to maintain a safe working environment.
- Meaning and relevance of various words and phrases associated with hazards inherent in oil and gas
- Flash point
- Vapour density
- Vapour pressure
- Flammability
- Fire triangle
- Flammable range
- Toxicity
- Skin irritant
- Carcinogenic properties
- Properties and hazards of various gases associated with the oil and gas industry
- Hydrogen
- Hydrogen sulphide (H2S)
- Methane
- Liquefied Petroleum Gas (LPG)
- Liquefied Natural Gas (LNG)
- Nitrogen
- Oxygen
- Properties and hazards of associated products and their control measures
- Anti-foaming agents and antiwetting agents
- Micro-biocides
- Corrosion preventatives
- Refrigerants
- Water/steam
- Mercaptans
- Drilling muds (drilling fluid)
- Low Specific Activity (LSA) sludges
Consequently, it is important to know the meaning and relevance of the common terms used throughout the industry in relation to hazards in the oil and gas industry, and the following information covers some of the basic terms used.
Meaning and relevance of various words and phrases associated with hazards inherent in oil and gas
Flash point
The flash point of a volatile liquid is the lowest temperature at which it can vaporize to form an ignitable mixture when mixed with air. Consequently, storing a fluid at a temperature below its flash point is an effective way of preventing ignitable vapours from forming.

The characteristics of a vapour are described using two terms. These are:
- Vapour density;
- Vapour pressure.
Let’s look at these two terms more closely.
Vapour density
Vapour density is the measurement of how dense a vapour is in comparison with air.
Comparing a vapour’s density with air indicates whether the vapour will rise or fall if it is released into the atmosphere. So, if we say air has a density of 1, then any vapour with a density below 1 will rise as it is lighter than air, and any vapour with a density above 1 will fall as it is heavier than air.
Propane, which is a type of Liquefied Petroleum Gas (LPG), has a density of 2.0, which makes it heavier than air. Consequently,when mixed with air, propane vapour will fall.
Whereas methane (liquefied natural gas) has a density of 0.717, which makes it lighter than air, so when mixed with air methane vapour will rise.
Consideration of vapour density is a vital factor in deciding where to position gas detection equipment, general ventilation requirements, etc.
Vapour pressure
The process of evaporation involves the molecules on the surface of a liquid. When the energy within these molecules is sufficient for those molecules to escape, they do so in the form of a vapour. This is known as vapour pressure.
Vapour pressure is measured in the standard units of pressure known as pascal (Pa). 1 Pascal is 1 newton per square metre.
The greater the vapour pressure, the faster this process takes place, which results in a greater concentration of vapour. A substance with a high vapour pressure at normal temperatures is often referred to as volatile.
Flammability
Vapour which is flammable presents the Risk Management Techniques used in the Oil and Gas Industriesrisk of an explosion. However, some vapours are more flammable than others and, as such, are categorized to indicate the level of risk involved.
The degree of flammability can be expressed as follows:
- Flammable;
- Highly flammable;
- Extremely flammable.
Let’s look at the definition of these categories more closely.
Flammable
This describes a product which is easily ignitable and capable of burning rapidly. Note that the word inflammable has the same meaning as flammable.
In the UK a flammable liquid is defined as a liquid that has a flash point of between 21 °C and 55 °C. However, in the USA there is a precise definition of flammable liquid as one with a flash point below 100 °F (37.8 °C).
Highly flammable
This is describing a product which has a flash point below 21 °C but which is not defined as extremely flammable.
Extremely flammable
This describes a product which has a flash point lower than 0 °C and a boiling point of 35 °C or lower.
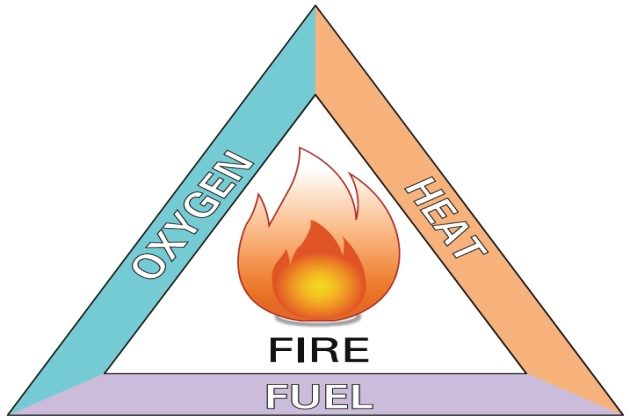
Fire triangle
For a fire to start, there are three elements which have to be present. These are:
- a source of fuel;
- a source of heat or ignition;
- oxygen.
This is known as the “fire triangle”.
If any one of these elements is eliminated, then a fire cannot continue or start.
Flammable range
Within the fire triangle where the source of fuel is a vapour, for it to burn it has to fall within a specific percentage range in comparison with air. If the mixture of flammable vapour and air has too much flammable vapour in it, the atmosphere will not burn. This is described as an atmosphere that is “too rich” to burn. Conversely, if the mixture of flammable vapour and air has too little flammable vapour in it, the atmosphere will again not burn. This is described as an atmosphere that is “too lean” to burn.
The lower flammable limit is the lowest concentration of a gas or vapour in air which is capable of being ignited.
The upper flammable limit is the highest concentration of a gas or vapour in air which is capable of being ignited.
The percentage of flammable vapour which falls between these two parameters (not too rich to burn but also not too lean to burn) is known as the flammable range.
For example, methane gas has a lower flammable limit of 4.4 per cent and an upper flammable limit of 15-17 per cent. This is the particular flammable range of methane gas, and at any point between these two limits the atmosphere is flammable.
There is always a risk of fire and explosion in an area which contains vapour within the flammable range. It is vital, therefore, to control the atmosphere to make sure that the flammable range is not reached. Purging (replacing) the air in storage tanks with nitrogen is such a control. This is because nitrogen is an inert gas (i.e. it will not burn).
Toxicity
Toxicity is used in two senses:
- To denote the capacity to cause harm to a living organism;
- To indicate the adverse effects caused by a chemical This information is available on the Material Safety Data Sheet which comes with any chemical.
With regards to toxicity, the following further descriptions may be used:
- Acute toxicity;
- Chronic toxicity.
Let’s look at the definitions of these terms more closely.
Acute toxicity is a term which describes the effect a substance has had on a person after either a single exposure or from several exposures within a short space of time (e. g. 24 hours or less). An example of this would be from radiation.
Chronic toxicity is a term which describes the effects a substance has had on a person after many exposures over a longer period of time (e. g. months or years). An example of this would be exposure to asbestos.
Skin irritant
The Occupational Health and Safety Administration (OSHA) describes skin irritant as “a chemical which is not corrosive, but which causes a reversible inflammatory effect on living tissue by chemical action at the site of contact”.
This means that the substance may cause a local inflammatory reaction of the skin exposed to it either immediately after one exposure or from repeated exposure. However, the inflammation is reversible.
Reactions can be strong or weak. Substances which cause a strong reaction may possibly be diluted in order to weaken the reaction.
The immediate reaction from strong irritants is called acute irritant contact dermatitis. On the other hand, it may take a number of exposures over a longer period of time for weak irritants to cause a reaction. This is known as chronic irritant contact dermatitis.
Carcinogenic properties
A carcinogen is defined as any substance that can cause, or aggravate, cancer. They fall into two groups:
- Genotoxic carcinogens;
- Non-genotoxic carcinogens.
Genotoxic carcinogens are those which react with DNA directly or with macromolecules which then react with DNA. There are no safe thresholds of exposure to genotoxic carcinogens.
Non-genotoxic carcinogens do not react directly with DNA although they do cause cancer in other ways. There may be some threshold exposure limits for substances which fall within this group.
Properties and hazards of various gases associated with the oil and gas industry
Hydrogen
Hydrogen is a gas which is difficult to detect as it is odourless and colourless. It is lighter than air (with a density of 0.07 when compared with air) and so will rise when released. Hydrogen is a highly flammable gas when it is mixed with air (flammable range 4-75 per cent) and it burns with an invisible flame. The only way to detect burning hydrogen is when it ignites something else which has a visible flame. Consequently, should hydrogen be suspected to be present within the atmosphere, it is essential to ensure there are no sources of ignition in the vicinity.
Hydrogen sulphide (H2S)
Hydrogen sulphide \( (H_2S) \) is produced from decaying vegetation and marine micro-organisms. It is a toxic, corrosive and flammable gas. It is a hazard for the oil and gas industry as it can be released as it comes to the surface with drilling shale.
Hydrogen sulphide \( (H_2S) \) is an extremely dangerous gas. It has a density of 1.39 when compared with air and tends to drift in low lying areas such as pits, cellars, drains, etc. As such, it is difficult to disperse.
Read also: All you need to know about Process Safety Management in Liquefied Petroleum Gas Industry
It is toxic when inhaled because, when it enters the bloodstream, it combines with the haemoglobin in red blood cells, preventing the absorption of oxygen into the blood, thus rapidly causing asphyxiation. It has an effect which is similar to that of carbon monoxide.
Although hydrogen sulphide has a foul odour, it very rapidly paralyses the sense of smell and can quickly overcome anyone exposed to it and asphyxiate them. Even very low concentrations of the gas can prove fatal. For this reason it has a Time Weighted Average (TWA) of 8 hours at 5 ppm, or 15 minutes at 10 ppm. These limits are those which are applicable in the UK as set by the UK Health and Safety Executive. However, international limits may vary.
Methane
Methane is an odourless, colourless gas which exists naturally in the substrate. It is lighter than air with a density of 0.717 compared with air. It is a flammable gas and, when mixed with air in concentrations between 5 and 15 per cent, is explosive.
Although methane is not toxic at low concentrations, it can cause asphyxiation if the level is high enough to reduce the amount of inhaled oxygen.
Liquefied Petroleum Gas (LPG)
Liquefied Petroleum Gas (LPG) is a mixture of hydrocarbon gases which are highly flammable and used as fuel in heating and cooking appliances and motor vehicles. It is also used as an aerosol propellant and refrigerant. The two main types of LPG are butane and propane.
LPG is an odourless, colourless gas which has a density of 2.0 when compared with air and tends to drift in low lying areas such as pits, cellars, drains, etc. As such, it is difficult to disperse.
Liquefied Petroleum Gas (LPG) expands at a rate of 250:1 at atmospheric pressure when it changes from a liquid to a gas. Consequently, it can cause a massive vapour cloud from a relatively small amount of liquid when that liquid is released into the air.
Another issue with liquefied petroleum gas (LPG) is that, to store it effectively, it has to be converted from a gas to a liquid, which means it is stored at a temperature of between 0 °C and -44 °C. Consequently, any moisture which settles to the bottom of the tank storing the LPG will need to be drained off. This operation is extremely hazardous as it carries the risk of this water freezing the drain valve in an open position and allowing LPG to escape.
This very scenario occurred in 1966 at Feyzin in France. The resulting Boiling Liquid Expanding Vapour Explosion (BLEVE) killed fifteen people and injured a further eighty-one.
Liquefied petroleum gas (LPG) is toxic and can cause:
- Asphyxiation;
- Cold burns to the skin on contact;
- Brittle fracture to carbon steel on contact;
- Environmental damage;
- Fire and explosion.
Liquefied Natural Gas (LNG)
Liquefied Natural Gas (LNG) is a colourless, odourless, highly flammable natural gas which is made up of methane (85-95 per cent), ethane, propane and butane. It is non-corrosive and non-toxic although, like methane, it can cause asphyxiation if the concentration is high enough when inhaled.
To convert natural gas from a gas to a liquid it needs to go through a process of condensation using liquid nitrogen. This reduces the temperature to -162 °C where it takes up 600 times less space in its liquid form than it does as a gas. The hazards associated with Liquefied Natural Gas (LNG) are:
- Asphyxiation;
- Cold burns to the skin on contact;
- Brittle fracture to carbon steel on contact;
- Fire and explosion.
Nitrogen
Nitrogen is the most abundant gas in the earth’s atmosphere, 78 per cent by volume. It is a colourless, odourless, non-flammable gas which is often used as a blanket gas in storage tanks and for purging equipment and processes of oxygen and hydrocarbons, thus eliminating the hazards of fire and explosion.
The primary hazard associated with nitrogen is asphyxiation when it is used in confined spaces to displace oxygen.
Oxygen
Oxygen is an odourless, colourless gas which is present in the atmosphere. It is vital for sustaining life as it is breathed in and absorbed into the bloodstream. It is considered to be a safe gas, but it can present the following hazards:
- Asphyxiation – this is because the body is stimulated to breathe by the level of carbon dioxide \( (CO_2) \) in the air and should a situation arise where oxygen is released into an area displacing the carbon dioxide, then the stimulus to breathe could cease, causing death by asphyxiation;
- It is one element of the “fire triangle”, i.e. it allows a fire to burn;
- It can oxidize metal, i.e. cause rusting. This is a very serious hazard, especially in the oil and gas industry where large amounts of the infrastructure are manufactured from carbon steel and are at risk of failure due to rusting. It is not always obvious where rusting occurs, especially if it is within areas that are impossible to see, e. g. inside hollow structures.
Properties and hazards of associated products and their control measures
Anti-foaming agents and antiwetting agents
Anti-foaming and anti-wetting agents are used to prevent foam forming or to break down foam that has already been created in a process liquid during any production processes. Foam can have a detrimental effect on product quality and production efficiency by slowing down the process. Some anti-foaming agents are oil based and others are silicone based.
Anti-wetting agents are coatings which are applied to surfaces of vulnerable components which are subject to moisture and subsequent corrosive activities. They are known as “hydrophobic” coatings, meaning they repel moisture.
Although both these types of agents are generally non¬hazardous, it is advisable to wash any areas of skin contact with soap and water. Information provided by the product’s Material Safety Data Sheet (MSDS) should always be consulted when they are used.
Micro-biocides
Micro-biocides are used to protect against the harmful effects of bacteria, e. g. legionella, which can proliferate in air conditioning systems and humidifiers. They do this by destroying the bacteria if it is present or by preventing its formation.
They are also used as corrosion inhibitors on some metals, e. g. steel pipelines.
Micro-biocides are classed as irritants to skin and eyes on contact, as well as being toxic if ingested.
Corrosion preventatives
Corrosion is a major hazard in the oil and gas industry. Preventing corrosive activity from causing damage to infrastructure is essential. Some corrosion preventatives come in the form of a water-displacing film which acts by spreading across the surface of metals, displacing water from cracks and crevices and forming a barrier to corrosive activity.
Other types of preventatives are applied to metals; they then dry to a hard resin or waxy film, thus forming a barrier to corrosive activity.
Information provided by the product’s Material Safety Data Sheet (MSDS) should always be consulted when they are used.
Refrigerants
Refrigerants are liquefied gas under pressure and pose a minimal risk provided they remain contained within their allotted systems. Problems arise when there is an escape or release of the refrigerant because it can displace oxygen in the atmosphere with the potential to provoke asphyxiation. To minimize the risk it is advisable to conduct regular safety checks on all equipment and systems, and have in place emergency procedures to deal with any unexpected release of the refrigerant.
Data relating to the refrigerant will be contained in the Material Safety Data Sheet (MSDS) which is provided with the product. This should state the toxicity status of the product as well as the safe exposure limits which apply.
The hazards associated with refrigerants are:
- Injury from components or material ejected by the high pressure escape;
- Frostbite injury to skin or eyes where contact with refrigerant is made;
- Asphyxiation;
- Possible explosion or fire if the refrigerant is flammable;
- When certain refrigerant gases burn they can produce other gases which can be very toxic;
- Liquid refrigerants have a very high expansion rate when changing from a liquid to a gas, causing overpressure;
- Refrigerant gases are heavier than air and will slump if the gases are accidently released.
Good practice and safe working procedures when dealing with refrigerants are as follows:
- Having procedures in place to deal with any unexpected release of refrigerant, e. g. recovery procedures and equipment to contain the refrigerant;
- Never working in confined spaces where there is a risk that refrigerants may be released. This is because of the very real risk of asphyxiation;
- Providing ventilation equipment to deal with any potentially high concentrations of refrigerant;
- Ensuring that anyone who is exposed to refrigerant gas is immediately moved out of the affected area to a place where they can breathe fresh air and be given oxygen as necessary. They will also need to be medically examined.
Water/steam
Water is used for cooling and dilution in process operations as well as for fighting fires, cleaning and within air conditioning systems. It does, however, present hazards, including:
- Legionella, which proliferates in air conditioning systems. Regular testing and maintenance systems can control this;
- Leptospira, which can be found in water which has its source in freshwater rivers or lakes. This can be transmitted to humans via broken skin or through the mucous membranes of the eyes, nose or mouth and, in extreme cases, can cause Weil’s disease, which can be fatal. Effective personal hygiene can control this;
- Corrosion, which attacks steel. Controls for this include applying a protective coating to steel components or fitting sacrificial anodes within the system to provide cathodic protection;
- The build-up of an electrostatic charge within pipework resulting in a potential explosion if this is discharged. The electrostatic charge can be caused by the friction of water flowing through the pipework. To avoid electrostatic charge build-up, the flow rate of water should be controlled at a suitable rate and the pipework should be bonded to earth;
- An increase in pressure within pipework and other components within a system. This may be caused by an increase in temperature during hydrostatic pressure testing, e. g. where there is exposure to direct sunlight. This may cause failure of the system with catastrophic effects.
Freezing water
Water expands when it freezes, and this can result in the failure (fracture) of pipework and/or other components within a system. Under certain conditions, plugs of ice (hydrates) can form and these can block pipes and pumps as well as preventing the closure of valves which, in critical situations, can have catastrophic effects.
A prime example of this was the Feyzin disaster in France in 1966, which we mentioned earlier. There, an operator was draining water from a pressurized propane tank when a hydrate plug formed in the drain valve. Consequently, he was unable to close the valve and a cloud of propane vapour escaped and exploded when it came into contact with a source of ignition.
Controls preventing this can be:
- Lagging pipes considered to be at risk of being damaged by freezing water;
- Fitting steam trace lines;
- Draining unused components.
Sea water
Sea water contains living organisms which can proliferate and cause blockages, e. g. in the heads of sprinkler systems. This can be avoided by the implementation of a regular maintenance programme to ensure all parts of a system are kept free from blockages, as well as using additives to kill any living organisms which may be present. A dry riser would also be a useful control.
Steam
Steam is used extensively in the oil and gas industry.
It is used to power turbines and generate electricity, as well as serving as a source of heat and/or energy to assist with many other operations and processes.
It can also be used to protect systems from the risk of freezing (tracer lines) and to serve as a heating system for areas where personnel are housed.
Steam has inherent risks as follows:
- It can cause thermal shock to a system if it is introduced into cold pipes or steam lines;
- It can cause failure of parts of a system if there is an uncontrolled expansion within it, e. g. jointed flanges could fail as they are some of the weakest points of the system;
- It can cause burns if anyone comes into contact with it.
Mercaptans
These are substances containing sulphur which are used to help detect natural gas by giving it an odour (natural gas in pure form is odourless). T-butyl mercaptan blends are used for this purpose as they smell of rotting cabbage, even in low concentrations in air.
Some mercaptans are harmful. For example, methyl mercaptan has the following hazards associated with it:
- It is harmful if inhaled;
- It is a respiratory irritant – chronic exposure may cause lung damage;
- It is a skin irritant;
- It is an eye irritant;
- It can depress the central nervous system;
- It has a flashpoint of -18 °C.
Drilling muds (drilling fluid)
When drilling operations are in progress, mud is pumped from the mud pits through the “drill string” where it is sprayed onto the drill bit. This allows for the cooling and cleaning of the drill bit throughout its operation.
It also allows the crushed rock cuttings, which have been drilled from the bore hole, to be carried to the surface where they are separated from the mud by the use of a “shale shaker” or other equipment before the mud is returned to the mud pit to be reused.
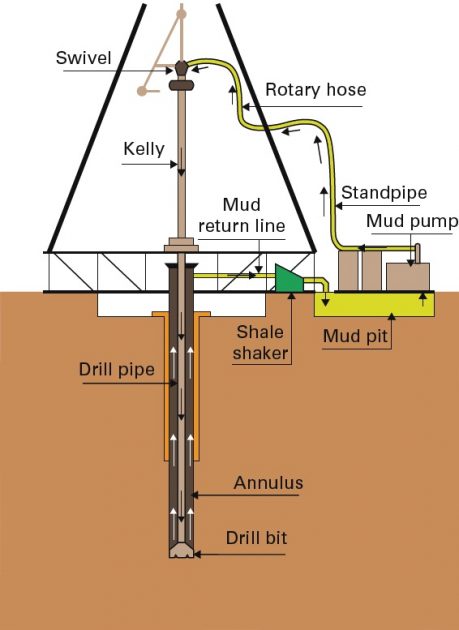
Crushed rock cuttings which are too small to be separated by the shale shaker will remain in the mud and be returned to the mud pit where they can settle to the bottom. These small cuttings are called “fines”.
Also, there may be natural gases or other flammable materials which have combined with the mud during the drilling operation. These have the ability to be released from the mud anywhere within the system where the mud is flowing back to the pit.
As a result of this there is risk of fire or explosion should these gases be exposed to a source of ignition. Control measures to prevent this include:
- Safe working procedures;
- Monitoring sensors;
- Equipment and wiring which has been certified as explosion proof.
Once the mud has been returned to the mud pit it is tested and, if necessary, further additives are combined with it to give it the required properties before it is pumped back into the system in a continuous cycle.
Functions of drilling mud
The main functions of drilling mud are as follows:
- Remove cuttings from the well;
- Suspend and release cuttings;
- Control formation pressures;
- Seal permeable formations;
- Maintain wellbore stability (line the walls of the hole);
- Minimize formation damage;
- Cool, lubricate and support the bit and drilling assembly;
- Transmit Questions and answers to Crew Evaluation System Test about Basic Hydraulic Systemhydraulic energy to tools and bit;
- Ensure adequate formation evaluation;
- Control corrosion (to an acceptable level);
- Facilitate cementing and completion.
There are different types of drilling fluids: water based, oil based and synthetic based muds.
We will now look in more detail at these three types of drilling fluid.
Drilling muds – Water Based Mud (WBM)
Water Based Mud (WBM) is a combination of clay and other additives blended with water to make a thick fluid. The more additives in the water, the thicker the mud will be. The fluid is normally made from indigenous clays although should particular muds be required the additives may be brought in from specialist suppliers.
Bentonite is a common additive which allows the mud to be fluid and free flowing when it is being pumped into the system and becomes semi-solid or gel-like when the pumping stops. Once pumping resumes it reverts to being free flowing.
Another additive which can be used is potassium formate, which allows the mud to have various other characteristics. These include:
- Questions and answers to Crew Evaluation System Test about Engine Oil ViscosityViscosity control;
- Shale stability;
- Enhancing the drilling rate of penetration;
- The cooling and lubricating of equipment.
Drilling muds – Oil Based Mud (OBM)
Oil Based Muds (OBMs) have, as their name suggests, oil – usually diesel oil – as their base fluid. The advantages oil based muds bring to the drilling process include:
- Increasing lubrication of the drill shaft;
- Enhancing shale inhibition;
- Adding greater cleaning ability.
OBMs allow for higher working temperatures to be used without adverse effects. However, there are environmental considerations to be taken into account when considering the use of OBMs.
Drilling muds – Synthetic Based Muds (SBMs)
These muds have the properties of oil based muds but have the advantage of being less toxic as their base is made from synthetic oil.
Low Specific Activity (LSA) sludges
The formations of rock and shale which contain oil and gas deposits also contain Naturally Occurring Radioactive Materials (NORM). These include:
- Uranium;
- Thorium;
- Radium;
- Lead-210.
Oil and gas were created in the earth’s crust by the decay of sea life in ancient seas and are, therefore, often found in aquifers which contain salt water (brine). Various minerals, as well as radioactive elements, are also dissolved in the brine and these separate out and form wastes at the surface. These include:
- Mineral scales inside pipes;
- Sludges;
- Contaminated equipment or components;
- Produced waters.
Workplace equipment where sludges might be found
The process of extraction exposes the environment and humans to the radioactive elements in the sludges. As such, they are classified as hazardous.
They can be found in the following locations:
- On the drill string;
- Inside vessels (demister pads);
- Inside filters;
- In coalescars (coarse filter/emulsifier);
- In coolers where tubes might be coated with sludge As we’ve mentioned, sludges are a mixture of liquid and suspended material and therefore present a range of hazards, for example:
- Skin irritant (possibly causing dermatitis);
- Inhalation (fumes or dust from dried sludges);
- Ingestion (poor hygiene, i.e. not washing, not cleaning up, eating at work site);
- Radiation;
- Carcinogenic;
- Environmental (pollutant);
- Absorption through the skin (dermatitis).
The main hazard of exposure to ionizing radiation from Low Specific Activity (LSA) materials is that of the inhalation and ingestion of radionuclides, especially of dust and fumes. Employees are at a higher risk of significant exposure to ionizing radiation if they work with dusty processes unless adequate control measures have been put in place to prevent the inhalation of dust.
Employees may be exposed, although to a lesser extent, to direct radiation where there is bulk storage of the material. They may also be exposed to external radiation if they are involved in cleaning operations or the dismantling of equipment which contains scale from oil and gas extractions.
Control measures that reduce risk to workers exposed to sludge
Employers should put in place controls to ensure that the risk of exposure to ionizing radiation is reduced “so far as is reasonably practicable”. The use of personal protective equipment may be used but only as a last resort after all other control measures have been considered. Engineering controls and the implementation of safe systems of work should take priority, and they include:
- The provision of ventilation equipment to contain dusts and fumes;
- The use of wet methods of working and good housekeeping to reduce the amount of dust in the atmosphere;
- Having equipment in place to collect sludge instead of using manual means;
- Diluting sludge with water;
- The use of permit-to-work systems – especially if the concentrations of dust or fumes reach a level where only designated or classified persons are allowed to work under the restrictions of a written safe system of work;
- The provision of training and awareness programmes;
- The provision of a health surveillance programme to monitor the health of employees;
- The use of Respiratory Protective Equipment (RPE) specifically chosen to protect against exposure to airborne radioactivity.
REVISION QUESTIONS FOR ELEMENT 1 CONTINUED
Answer 1
Vapour pressure is the process of evaporation where the energy within those molecules at the surface of a liquid is sufficient for those molecules to escape in the form of a vapour. Vapour density, on the other hand, is the measurement of how dense a vapour is in comparison with air.
Answer 2
Knowing the density of a gas/vapour will determine whether the gas/vapour will rise or fall if it escapes. This will help in determining where any detectors and sources of ventilation should be placed so that any build-up of hazardous gas/vapour can be detected quickly and dealt with appropriately.
Answer 3
- Injury from components or material ejected by the high pressure escape.
- Frostbite injury to skin or eyes where contact with refrigerant is made.
- Asphyxiation.
- Possible explosion or fire if the refrigerant is flammable.
- When certain refrigerant gases burn they can produce other toxic gases.
- Liquid refrigerants have a very high expansion rate when changing from a liquid to a gas causing overpressure.
- Refrigerant gases are heavier than air and will slump if the gases are accidently released resulting in potential pockets of gas in voids, drains, ducts, etc.
Answer 4
- Have pre-arranged procedures in place to deal with any unexpected release of refrigerant.
- Ensure personnel do not have to work in confined spaces where there is a risk that refrigerants may be released. This is because of the very real risk of asphyxiation.
- Provide ventilation equipment to deal with any potentially high concentrations of refrigerant.
- Ensure that procedures are in place that ensure anyone who is exposed to refrigerant gas is immediately moved out of the affected area to a place where they can breathe fresh air and be given oxygen as necessary. They will also need to be medically examined.
