This article describes the properties and hazards of transporting natural gas and its varieties.
- Types of natural gas carriers
- LPG
- LNG
- NGL
- LEG
- Ammonia NH3
- Chlorine Cl2
- Chemical gases
- Cargo properties
- States of matter
- Saturated vapour pressure
- Liquid and vapour densities
- Flammability and explosion
- Saturated hydrocarbons
- Unsaturated hydrocarbons
- Chemical gases
- Toxicity
- Threshold Limit Values (TLV)
- Methods of liquefaction
- Evaporation
- Boiling
- Condensation
- Distillation
- Saturated, Unsaturated or Superheated Steam
- Hazards from liquefied gas
- Flammability
- Toxicity
- Asphyxia
- Anaesthesia
- Frostbite
- Comparison of hazards in liquefied gas carriage and in the transport of normal petroleum
Types of natural gas carriers
IMO divides liquefied gases into the following groups:
- LPG – Liquefied Petroleum Gas;
- LNG – Liquefied Natural Gas;
- LEG – Liquefied Ethylene Gas;
- NH3 – Ammonia;
- Cl2 – Chlorine;
- Chemical gases.
The IMO gas carrier code define liquefied gases as gases with vapour pressure higher than 2,8 bar with temperature of 37,8 °C.
IMO gas code chapter 19 defines which products that are liquefied gases and have to be transported with gas carriers. Some products have vapour pressure less than 2,8 bar at 37,8 °C, but are defined as liquefied gases and have to be transported according to chapter 19 in IMO gas code. Propylene oxide and ethylene oxides are defined as liquefied gases. Ethylene oxide has a vapour pressure of 2,7 bar at 37,8 °C. To control temperature on ethylene oxide we must utilise indirect cargo cooling plants.
Products not calculated as condensed gas, but still must be transported on gas carriers, are specified in:
- IMO’s gas code;
- IMO’s chemical code.
The reason for transportation of non-condensed gases on gas carriers is that the products must have temperature control during transport because reactions from too high temperature can occur.
Condensed gases are transported on gas carriers either by atmospheric pressure (fully cooled) less than 0,7 bars, intermediate pressure (temperature controlled) 0,5 bars to 11 bars, or by full pressure (surrounding temperature) larger than 11 bars. It is the strength and construction of the cargo tank that is conclusive to what over pressure the gas can be transported.
LPG
LPG – Liquefied Petroleum Gas is a definition of gases produced by wet gas or raw oil. The LPG gases are taken out of the raw oil during refining, or from natural gas separation. LPG gases are defined as propane, butane and a mixture of these. Large atmospheric pressure gas carriers carry most of the LPG transported at sea. However, some LPG is transported with intermediate pressure gas carriers. Fully pressurised gas carriers mainly handle coastal trade. LPG can be cooled with water, and most LPG carriers have direct cargo cooling plants that condenses the gas against water.
The sea transport of LPG is mainly from The Persian Gulf to Japan and Korea. It is also from the north-west Europe to USA, and from the western Mediterranean to USA and Northwest Europe.
LPG is utilized for energy purposes and in the petro-chemical industry
LNG
LNG – Liquefied Natural Gas is a gas that is naturally in the earth. Mainly LNG contains Methane, but also contains:
- Ethane;
- Propane;
- Butane etc.
About 95 % of all LNG are transported in pipelines from the gas fields to shore, for example, gas pipes from the oil fields in the North Sea and down to Italy and Spain. Gas carriers transport the remaining 5 %. When LNG is transported on gas carriers, the ROB and boil off from the cargo is utilized as fuel for propulsion of the vessel. Cargo cooling plants for large LNG carriers are very large and expensive, and they will use a lot of energy. Small LNG carriers have cargo-cooling plants, and can also be utilized for LPG transportation.
The sea transport of LNG is from the Persian Gulf and Indonesia to Japan, Korea and from the Mediterranean to Northwest Europe and the East Coast of USA and from Alaska to the Far East.
LNG is used for energy purposes and in the petro-chemical industry.
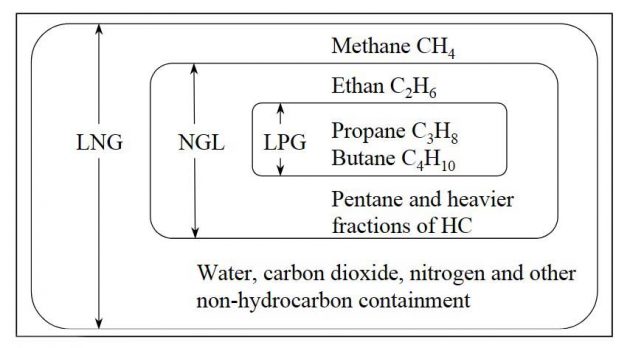
NGL
NGL – Natural Gas Liquid or wet gas is dissolved gas that exists in raw oil. The gas separates by refining raw oil. The composition of wet gas varies from oil field to oil filed. The wet gas consists of:
- Ethane;
- LPG;
- Pentane and heavier fractions of hydrocarbons or a mixture of these.
Atmospheric pressure gas carriers and semi-pressurized gas carriers carry the most of the wet gas.
Ethane can only be transported by semi-pressurized gas carriers, which have direct cascade cooling plants and are allowed to carry cargo down to -104 °C. This is because Ethane has a boiling point at atmospheric pressure of -89 °C. This will create too high condense pressure if using water as cooling medium. The cargo is condensed against Freon R22 or another cooling medium with boiling point at atmospheric pressure lower than -20 °C.
Wet gas is transported from the Persian Gulf to the East, Europe to USA and some within Europe. There is also some transport of wet gas in the Caribbean to South America.
NGL is utilized for energy purposes and in the petro-chemical industry.
LEG
LEG – Liquefied Ethylene Gas. This gas is not a natural product, but is produced by cracked wet gas, such as, Ethane, Propane, and Butane or from Naphtha. Ethylene has a boiling point at atmospheric pressure of -103,8 °C, and therefore has been transported in gas carriers equipped with cargo compartment that can bear such a low temperature. Cascade plants are used to condense Ethylene. As critical temperature of Ethylene is 9,7 °C one cannot utilize water to condense Ethylene. The definition of Ethylene tankers is LPG/LEG carrier.
Ethylene is very flammable and has a flammable limit from 2,5 % to 34 % by volume mixed with air. There are stringent demands regarding the oxygen content in Ethylene. The volume of ethylene must be less than 2 % in the gas mixture to keep the mixture below the LEL “lower explosion limit“. Normally, there are demands for less than 0,2 % oxygen in the gas mixture in order to prevent pollution of the cargo.
Ethylene is utilized as raw material for plastic and synthetic fibres.
Ethylene is transported from the Persian Gulf to the East, the Mediterranean to the East and Europe, the Caribbean to South America. There is also transport of Ethylene between the countries Malaysia, Indonesia and Korea
Ammonia NH3
The next gas we will focus on is Ammonia, which is produced by combustion of hydrogen and nitrogen under large pressure. Ammonia is a poisonous and irritating gas, it has TLV of 25 ppm and the odour threshold is on 20 ppm. It responds to water and there are special rules for vessels that transport Ammonia. We can locate the rules in the IMO Gas Code, chapters 14, 17 and 19.
When ammonia gas is mixed with water, a decreased pressure is formed by 1 volume part water absorbing 200 volume parts ammonia vapour. A decreased tank pressure will occur if there is water in the tank when commence loading ammonia and the tank hatch is closed. With an open hatch, we can replace the volume, originally taken up by the ammonia gas, with air.
One must not mix ammonia with alloys:
- copper;
- aluminium;
- zinc;
- nor galvanized surfaces.
Inert gas that contains carbon dioxide must not be used to purge ammonia, as these results in a carbamate formation with the ammonia. Ammonium carbamate is a powder and can blockage lines, valves and other equipment.
The boiling point for ammonia at atmospheric pressure is -33 °C, and must be transported at a temperature colder than -20 °C. One can cool ammonia with all types of cargo cooling plants. Ammonia is transported with atmospheric pressure gas carriers or semi-pressurised gas carriers. Gas carriers carrying Ammonia must be constructed and certified in accordance with IMO’s IGC code for transportation of liquefied gases. The definition for ammonia tanker is LPG/NH, carrier.
Ammonia is utilized as raw material for:
- the fertilizer industry;
- plastic;
- explosives;
- colours and detergents.
There is a lot of transportation from the Black Sea to USA, from USA to South Africa and from Venezuela to Chile.
Chlorine Cl2
Chlorine is a very toxic gas that can be produced by the dissolution of sodium chloride in electrolysis. Because of the toxicity of Chlorine, it is therefore transported in small quantities, and must not be transported in a larger quantity than 1 200 m3. The gas carrier carrying chlorine must be type 1G with independent type C tanks. That means the cargo tank must, at the least, lie B/5 “Breadth/5” up to 11,5 meter from the ships side. To transport Chlorine, the requirements of IMO IGC code, chapters 14, 17 and 19 must be fulfilled. Cooling of Chlorine requires indirect cargo cooling plants.
The difference of Chlorine and other gases transported is that Chlorine is not flammable.
Chlorine is utilized in producing chemicals and as bleaching agent in the cellulose industry.
Chemical gases
The chemical gases mentioned here is the gases produced chemically and are defined in IMO’s rules as condensed gases. Because of the gases boiling point at atmospheric pressure and special requirements for temperature control, these gases must be carried on gas carriers as specified by the IMO gas code. Condensed gases are liquids with a vapour pressure above 2,8 bars at 37,8 °C. Chemical gases that are mostly transported are Ethylene, Propylene, butadiene and VCM. Chemical gases that have to be transported by gas carriers are those mentioned in chapter 19 in IMO IGC code.
There are, at all times, stringent demands for low oxygen content in the cargo tank atmosphere, often below 0,2 % by volume. This involves that we have to use nitrogen to purge out air from the cargo compartment before loading those products.
In addition, even though the vapour pressure does not exceed 2,8 bars at 37,8 °C such as, ethylene oxide and propylene oxide or a mixture of these, they are still in the IMO gas code as condensed gases. Gas carriers that are allowed to transport ethylene oxide or propylene oxide must be specially certified for this. Ethylene oxide and propylene oxide have a boiling point at atmospheric pressure of respectively 11 °C and 34 °C and are therefore difficult to transport on tankers without indirect cargo cooling plants. Ethylene oxide and propylene oxide cannot be exposed to high temperature and can therefore not be compressed in a direct cargo cooling plant. Ethylene oxide must be transported on gas tanker type 1G. Chemical gases like propylene, butadiene and VCM are transported with medium-sized atmospheric pressure tankers from 12 000 m3 to 56 000 m3. Semi-pressurized gas carriers are also used in chemical gas trade and then in smaller quantity as from 2 500 m3 to 15 000 m3.
Chemical gases are transported all over the world, and especially to the Far East where there is a large growth in the petro-chemical industry. Chemical gases are mainly utilized in the petro-chemical industry and rubber production.
Cargo properties
States of matter
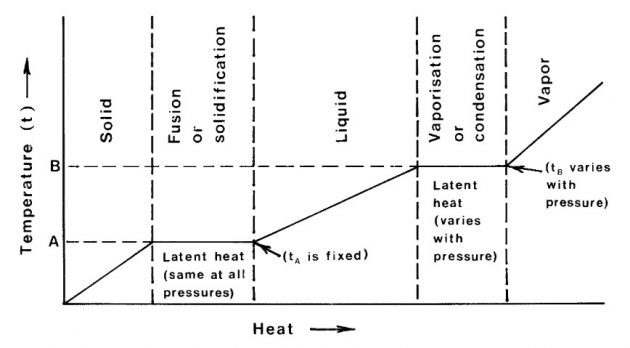
Most substances can exist in either the solid, liquid or vapour state. In changing from solid to liquid (fusion) or from liquid to vapour (vaporisation), heat must be given to the substance. Similarly, in changing from vapour to liquid (condensation) or from liquid to solid (solidification), the substance must give up heat. The heat given to or given up by the substance in changing state is called latent heat. For a given mass of the substance, the latent heats of fusion and solidification are the same.
Similarly, latent heats of vaporisation and of condensation are the same, although different from the latent heat of fusion or solidification. Fusion or solidification occurs at a specific temperature for the substance and this temperature is virtually independent of the pressure. Vaporisation or condensation of a pure substance, however, occurs at a temperature which varies widely dependent upon the pressure exerted on the substance. The latent heat of vaporisation also varies with pressure.
Picture 1 illustrates these temperature/heat relationships as a substance is heated or cooled through its three states; the temperatures of fusion or solidification (A) and of vaporisation or condensation (B) are all well defined.
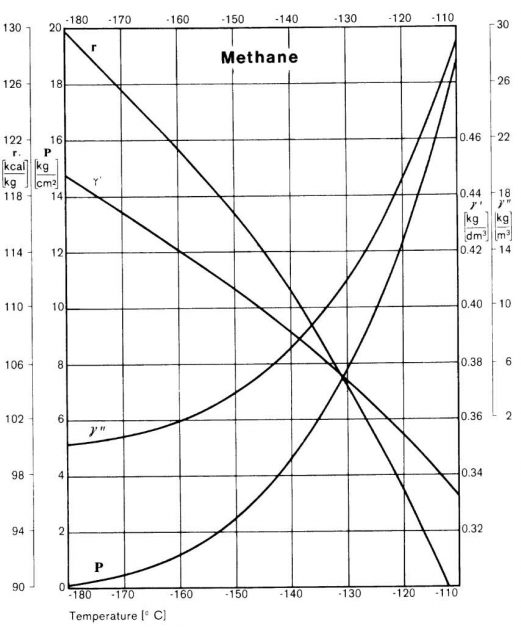
For liquefied cases, we are not concerned with the solid state since this can only occur at temperatures well below those at which the liquefied gas is carried. Temperatures, pressures and latent heats of vaporisation, however, are of fundamental importance. This data may be presented in graphical form such as Picture 2 which gives curves for vapour pressure, liquid density, saturated vapour density and latent heat of vaporisation against temperature for methane. Similar graphical presentation of these properties are available for all the principal liquefied gases carried by sea and some of these presentations are reproduced in the Data Sheets of Appendix 1 of the ICS Tanker Safety Guide (Liquefied Gas).
It is convenient here, against the background of the preceding, paragraphs, to consider what happens when a liquefied gas is spilled. Firstly, consider the escape from its containment of a fully refrigerated liquid. The liquid is already at or near atmospheric pressure but, on escape, it is inevitably brought immediately into contact with objects such as structures, the ground or the sea, which are at ambient temperature. The temperature difference between the cold liquid and the objects it contacts provides an immediate transfer of latent heat to the liquid, resulting in rapid evolution of vapour.
The abstraction of heat from contacted solid objects cools them, reducing the temperature difference and stabilising the rate of evaporation to a lower level than initially until the liquid is completely evaporated. In the case of spillage on to water, the convection in the upper layers of the water may largely maintain the initial temperature difference and evaporation may continue at the higher initial rate. Spillage from a pressurized container is initially different in that the liquid on escape is at a temperature not greatly different from ambient temperature but the liquid is released from its containment pressure down to ambient pressure.
Extremely rapid vaporisation ensues, the necessary latent heat being taken primarily from the liquid itself which rapidly cools to its temperature of vaporisation at atmospheric pressure. This is called flash evaporation and, depending upon the change in pressure as the liquid escapes from its containment, a large proportion of the liquid may flash off in this way.
The considerable volume of vapour produced within the escaping liquid causes the liquid to fragment into small droplets. Depending upon the change in pressure as the liquid escapes, these droplets will be ejected with a considerable velocity. These droplets take heat from the surrounding air and condense the water vapour in the air to form a white visible cloud and vaporise to gas in this process. Thereafter any liquid which remains will evaporate in the same way as for spilled fully refrigerated liquid until the spillage is wholly vaporised. Apart from the hazards introduced by the generation of vapour which will become flammable as it is diluted with the surrounding air, the rapid cooling imposed upon contacted objects will cause cold burns on human tissue and may convert metallic structure to a brittle state.
Saturated vapour pressure
Vapour in the space above a liquid is not static since liquid molecules near the surface are constantly leaving to enter the vapour phase and vapour molecules are returning to the liquid phase. The space is said to be unsaturated with vapour at a particular temperature if the space can accept more vapour from the liquid at that temperature. A saturated vapour at any temperature is a vapour in equilibrium with its liquid at that temperature. In that condition the space cannot accept any further vapour from the liquid, although a continuous exchange of molecule, between vapour and liquid takes place.
The pressure exerted by a saturated vapour at a particular temperature is called the saturated vapour pressure of that substance at that temperature. Various methods exist for measurement of saturated vapour pressures and one is illustrated in Picture 3. This apparatus consists of a barometer tube (C) which is filled with mercury, inverted and immersed in a mercury reservoir (A). The space above the mercury is a vacuum (B) though not perfect because of the presence of mercury vapour in that space. The height of mercury (X) is a measure of atmospheric pressure. A small amount of the liquid under test is introduced into the mercury barometer and rises to the vacuum space where it immediately vaporises and exerts a vapour pressure. This vapour pressure pushes the mercury down in the barometer tube to a new level (Y). The saturated vapour pressure exerted by a test liquid is the difference between the heights of the mercury column X and Y, usually expressed in mm of mercury.
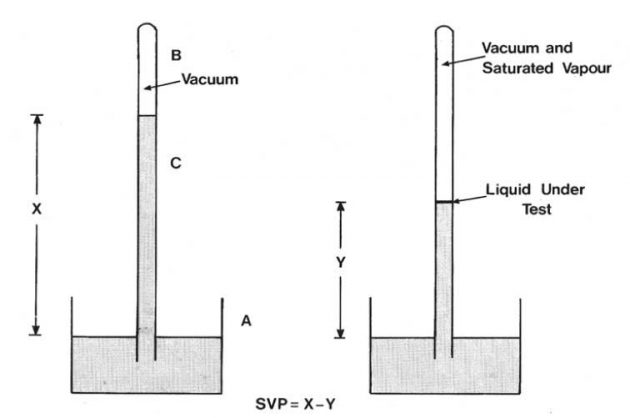
If the mercury column containing the small amount of liquid under test is now suitably heated, then the mercury level will fall indicating that the saturated vapour pressure has increased with increasing temperature. It is possible by this means to determine the saturated vapour pressure for the liquid under test at various temperatures.
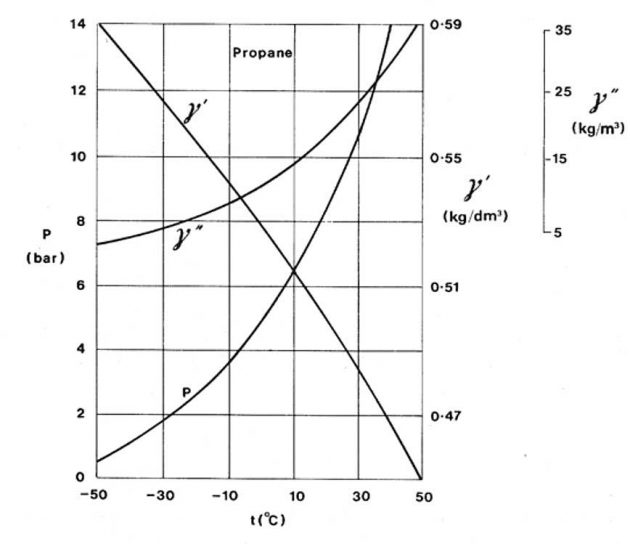
Whereas evaporation is a surface phenomenon where the faster moving molecules escape from the surface of the liquid, boiling takes place in the body of the liquid when the vapour pressure is equal to the pressure in the liquid. By varying the pressure above the liquid it is possible to boil the liquid at different temperatures. Decreasing the pressure above the liquid lowers the boiling point and increasing the pressure raises the boiling point. The curve marked P on Picture 4 illustrates the variation in saturated vapour pressure with temperature for propane. It will be noticed that an increase in the temperature of the liquid causes a nonlinear increase in the saturated vapour pressure. Also shown on Picture 4 are the variations of propane liquid densities and saturated vapour densities with temperature.
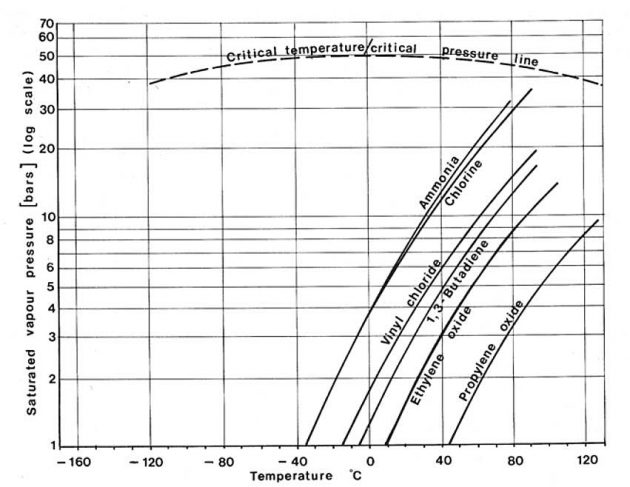
Different liquefied gases exert different vapour pressures as can be seen from Pictures 5 and 6. The vertical axis in these two figures gives the saturated vapour pressure on a logarithmic scale which changes the shape of the curves from that of P in Picture 4. Picture 5 shows that for the hydrocarbon gases, smaller molecules exert greater vapour pressures than large ones. In general, the chemical gases shown in Picture 6 exert much lower saturated vapour pressures than the small hydrocarbon molecules.
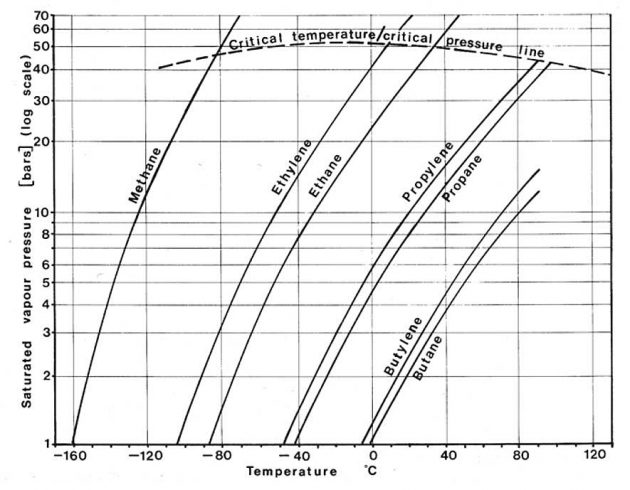
The point of intersection of these curves with the horizontal axis indicates the atmospheric boiling point of the liquid (the temperature at which the saturated vapour pressure is equal to atmospheric pressure). This is the temperature at which these cargoes would be transported in a fully refrigerated containment system.
Whereas the bar is now the most frequently used unit in the gas industry for the measurement of pressure, other units such as kgf/cm2, atmospheres or millimetres of mercury are frequently encountered.
All gauges used for the measurement of pressure measure pressure difference. Gauge pressure is therefore the pressure difference between the pressure to which the gauge is connected and the pressure surrounding the gauge. The absolute value of the pressure being measured is obtained by adding the external pressure to the gauge pressure.
Vapour pressures, though they may be often determined by means of a pressure gauge, are a fundamental characteristic of the liquid and are essentially absolute pressures. Tank design pressures and relief valve settings, however, like pressure gauge indications, are physically the differences between internal and external pressure and thus are gauge pressures. For consistency throughout this book all such pressures are given in bars but to avoid confusion the unit is denoted as “barg” where a gauge pressure is intended.
- A liquefied gas has been defined in terms of its vapour pressure as being a substance whose vapour pressure at 37,8 ℃ is equal to or greater than 2,8 bar absolute (IMO definition).
Liquid and vapour densities
The density of a liquid is defined as the mass per unit volume and is commonly measured in kilograms per decimetre cubed (kg/dm3). Alternatively, liquid density may be quoted in kg/liter or in kg/m3. The variation with temperature of the density of a liquefied gas in equilibrium with its vapour is shown for propane in curve y′ of Picture 4. As can be seen, the liquid density decreases markedly with increasing temperature. This is due to the comparatively large coefficient of volume expansion of liquefied gases. All the liquefied gases, with the exception of chlorine, have liquid relative densities less than one. This means that in the event of a spillage onto water these liquids would float prior to evaporation.
| Table 1. Conversion factors for units of pressure | ||||||
|---|---|---|---|---|---|---|
| Bar | Atmosphere (Atm) | kgf/cm2 | mm Hg (torr) | mm H20 | pascal (Pa) | |
| 1 Bar | 1,00 | 0,987 | 1,020 | 750 | 1,021×104 | 1,00×105 |
| 1 Atmosphere | 1,013 | 1,00 | 1,033 | 760 | 1,035×104 | 1,013×105 |
| 1 kgf/cm2 | 0,981 | 0,968 | 1,00 | 735,6 | 1,001×104 | 0,981×105 |
| 1 mm Hg (torr) | 1,33×10-3 | 1,316×10-3 | 1,360×10-3 | 1,00 | 13,62 | 133,3 |
| 1 mm H20 | 0,980×10-4 | 0,967×1014 | 9,98×10-5 | 7,342×10-2 | 1,00 | 9,80 |
| 1 pascal (Pa) | 1,00×10-5 | 0,987×10-5 | 1,02×10-5 | 7,501×10-3 | 0,102 | 1,00 |
The variation of the density of the saturated vapour of liquefied propane with temperature is given by curve y′′ of Figure 4. The density of vapour is commonly quoted in units of kilograms per cubic meter (kg/m′). The density of the saturated vapour increases with increasing temperature. This is because the vapour is in contact with its liquid and as the temperature rises more liquid transfers into the vapour phase in order to provide the increase in vapour pressure. This results in a considerable increase in mass per unit volume of the vapour space.
All the liquefied gases produce vapours which have a relative vapour density greater than one with the exceptions of methane (at temperatures greater than -100 °C). Vapours released to the atmosphere and which are denser than air tend to seek lower ground and do not disperse readily.
Flammability and explosion
Combustion is a chemical reaction, initiated by a source of ignition, in which a flammable vapour combines with oxygen in suitable proportions to produce carbon dioxide, water vapour and heat. Under ideal conditions the reaction for propane can be written as follows:
Under certain circumstances when, for example, the oxygen supply to the source of fuel is restricted, carbon monoxide or carbon can also be produced.
The three requirements for combustion to take place are:
- fuel;
- oxygen;
- ignition.
The proportions of flammable vapour to oxygen or to air must be within the flammable limits.
The gases produced by combustion are heated by the combustion reaction. In open, unconfined spaces the consequent expansion of these gases is unrestricted and the combustion reaction may proceed smoothly without undue overpressures developing. If the free expansion of the hot gases is restricted in any way, pressures will rise and the speed of flame travel will increase, depending upon the degree of confinement encountered.
Increased flame speed in turn gives rise to more rapid increase in pressure with the result that damaging overpressures may be produced and, even in the open, if the confinement resulting from surrounding pipework, plant and buildings is sufficient, the combustion can take on the nature of an explosion. In severely confined conditions, as within a building or ship’s tank where the expanding gases cannot be adequately relieved, the internal pressure and its rate of increase may be such as to disrupt the containment. Here, the resultant explosion is not so much directly due to high combustion rates and flame speed as to the violent expulsion of the contained high pressure upon containment rupture.
The boiling liquid expanding vapour explosion (BLEVE) is a phenomenon associated with the sudden and catastrophic failure of the pressurized containment of flammable liquids in the presence of a surrounding fire. Such incidents have occurred with damaged rail tank car or road tank vehicle pressure vessels subject to intense heat from surrounding fire.
This heat has increased the internal pressure and, particularly at that part of the vessel not wetted by liquid product, the vessel’s structure is weakened to the point of failure. The sudden release of the vessel’s contents to atmosphere and the immediate ignition of the resultant rapidly expanding vapour cloud have produced destructive overpressures and heat radiation. There have been no instances of this kind, nor are they likely to occur, with the pressure cargo tanks on liquefied gas tankers where, by requirement, pressure relief valves are sized to cope with surrounding fire, tanks are provided with water sprays and general design greatly minimises the possibilities of a surrounding fire occurring.
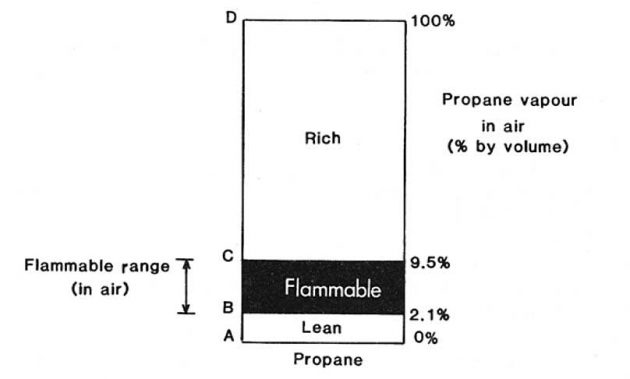
The term flammable range gives a measure of the proportions of flammable vapour to air necessary for combustion to be possible. The flammable range is the range between the minimum and maximum concentrations of vapour (% by volume) in air, which form a flammable mixture. These terms are usually abbreviated to LFL (lower flammable limit) and UFL (upper flammable limit). This concept is illustrated for propane in Picture 8.
All the liquefied gases, with the exception of chlorine, are flammable but the values of the flammable range are variable and depend on the particular vapour. These are listed in Table 2. The flammable range of a particular vapour is broadened in the presence of oxygen in excess of that normally in air; the lower flammable limit is not much affected whereas the upper flammable limit is considerably raised. All flammable vapours exhibit this property and as a result oxygen should not normally be introduced into an atmosphere where flammable vapours exist. The oxygen cylinders associated with oxyacetylene burners and oxygen resuscitators should only he introduced into hazardous areas under strictly controlled conditions.
The flash point of a liquid is the lowest temperature at which that liquid will evolve sufficient vapour to form a flammable mixture with air. High vapour pressure liquids such as liquefied gases have extremely low flash points, as seen from Picture 7. However, although liquefied gases are never carried at temperatures below their flash point, the vapour spaces above such cargoes are non-flammable since they are virtually 100 % rich with cargo vapour and are thus far above the upper flammable limit.
| Table 2. Ignition properties for liquefied gases | |||
|---|---|---|---|
| Liquefied gas | Flash point (°C) | Flammable range (% by vol. in air) | Auto-ignition temperature (°C) |
| Methane | -175 | 5,3-14 | 595 |
| Ethane | -125 | 3,1-12,5 | 510 |
| Propane | -105 | 2,1-9,5 | 468 |
| n-Butane | -60 | 1,8-8,5 | 365 |
| i-Butane | -76 | 1,8-8,5 | 500 |
| Ethylene | -150 | 3-32 | 453 |
| Propylene | -180 | 2-11,1 | 453 |
| α-Butylene | -80 | 1,6-9,3 | 440 |
| β-Butylene | -72 | 1,8-8,8 | 465 |
| Butadiene | -60 | 2-12,6 | 418 |
| Isoprene | -50 | 1-9,7 | 220 |
| VCM | -78 | 4-33 | 472 |
| Ethylene oxide | -18 | 3-100 | 429 |
| Propylene oxide | -37 | 2,8-37 | 465 |
| Ammonia | -57 | 16-25 | 615 |
| Chlorine | Non-flammable | ||
The auto-ignition temperature of a substance is the temperature to which its vapour in air must be heated for it to ignite spontaneously. The auto-ignition temperature is not related to the vapour pressure or to the flash point of the substance and, since most ignition sources in practice are external flames or sparks, it is the flash point rather than the autoignition characteristics of a substance which is generally used for the flammability classification of hazardous materials. Nevertheless, in terms of the ignition of escaping vapour by steam pipes or other hot surfaces, the auto-ignition temperature of vapours of liquefied gases are worthy of note and are also listed in Table 2.
| Table 3. Flammability range in air/oxygen for various liquefied gases | ||
|---|---|---|
| Flammable range (% by volume) | ||
| (in air) | (in oxygen) | |
| Propane | 2,1-9,5 | 2,1-55,0 |
| n-Butane | 1,8-8,5 | 1,8-49,0 |
| VCM | 4,0-33,0 | 4,0-70,0 |
Should a liquefied gas be spilled in an open space, the liquid will rapidly evaporate to produce a vapour cloud which will be gradually dispersed downwind. The vapour cloud or plume would be flammable only over part of its downwind travel. The situation is illustrated in general terms in Picture 8.
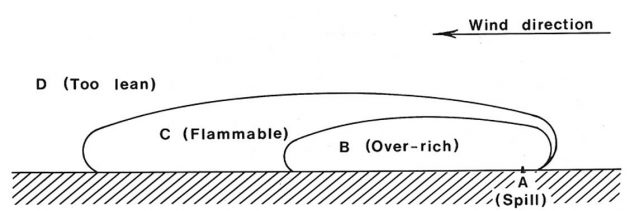
The region B immediately adjacent to the spill area A would be non-flammable because it is over-rich, i.e. it contains too low a percentage of oxygen to be flammable. Region D would also be non-flammable because it is too lean, i.e. it contains too little vapour to be flammable. The flammable zone would be between these two regions as indicated by C.
Saturated hydrocarbons
The saturated hydrocarbons methane, ethane, propane and butane are all colourless and odourless liquids under normal conditions of carriage.
They are all flammable gases and will burn in air and/or oxygen to produce carbon dioxide and water vapour. As they are chemically nonreactive they do not present chemical compatibility problems with materials commonly used in handling. In the presence of moisture, however, the saturated hydrocarbons may form hydrates.
Sulphur compounds such as mercaptans are often added as odorizes prior to sale to aid in the detection of these vapours. This process is referred to as “stenching“.
Unsaturated hydrocarbons
The unsaturated hydrocarbons ethylene, propylene, butylene, butadiene and isoprene are colourless liquids with a faint, sweetish characteristic odour. They are, like the saturated hydrocarbons, all flammable in air and/or oxygen, producing carbon dioxide and water vapour. They are chemically more reactive than the saturated hydrocarbons and may react dangerously with chlorine.
Ethylene, propylene and butylene do not present chemical compatibility problems with materials of construction, whereas butadiene and isoprene, each having two pairs of double bonds, are by far the most chemically reactive within this family group. They may react with air to form peroxides which are unstable and tend to induce polymerisation. Butadiene is incompatible in the chemical sense with:
- copper;
- silver;
- mercury;
- magnesium and aluminium.
Butadiene streams often contain traces of acetylene, which can react to form explosive acetylides with brass and copper.
Water is soluble in butadiene, particularly at elevated temperatures and Picture 9 illustrates this effect. The figures quoted are for the purpose of illustration only. On cooling water-saturated butadiene the solubility of the water decreases and water will separate out as droplets, which will settle as a layer in the bottom of the tank. For instance, on cooling water- saturated butadiene from +15 °C to +5 °C approximately 100 ppm of free water would separate out.
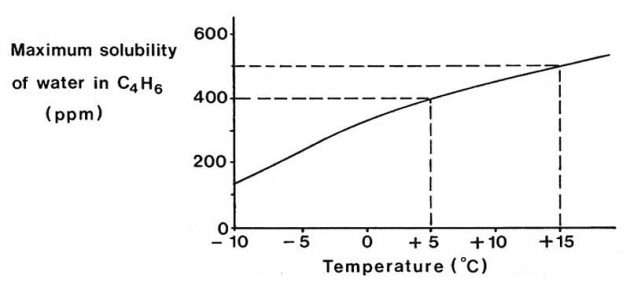
On this basis, for a 1 000 m3 tank, 100 dm3 of free water would require to be drained from the bottom of the tank. On further cooling to below 0 °C this layer of water would increase in depth and freeze.
Chemical gases
The chemical gases commonly transported in liquefied gas carriers are:
- ammonia;
- vinyl chloride monomer;
- ethylene oxide;
- propylene oxide and chlorine.
Since these gases do not belong to one particular family their chemical properties vary.
Liquid ammonia is a colourless alkaline liquid with a pungent odour. The vapours of ammonia are flammable and burn with a yellow flame forming water vapour and nitrogen, however, the vapour in air requires a high concentration (16-25 %) to be flammable, has a high ignition energy requirement (600 times that for propane) and burns with low combustion energy. For these reasons the IMO Codes, while requiring full attention to the avoidance of ignition sources, do not require flammable gas detection in the hold or interbarrier spaces of carrying ships.
Nevertheless, ammonia must always be regarded as a flammable cargo.
Ammonia is also toxic and highly reactive. It can form explosive compounds with:
- mercury;
- chlorine;
- iodine;
- bromine;
- calcium;
- silver oxide and silver hypochlorite.
Ammonia vapour is extremely soluble in water and will be absorbed rapidly and exothermically to produce a strongly alkaline solution of ammonium hydroxide. One volume of water will absorb approximately 200 volumes of ammonia vapour. For this reason, it is extremely undesirable to introduce water into a tank containing ammonia vapour as this can result in a vacuum condition rapidly developing within the tank.
Since ammonia is alkaline, ammonia vapour/air mixtures may cause stress corrosion. Because of its highly reactive nature copper alloys, aluminium alloys, galvanised surfaces, polyvinyl chloride, polyesters and viton rubbers are unsuitable for ammonia service. Mild steel, stainless steel, neoprene rubber and polythene are, however, suitable.
Vinyl chloride monomer (VCM) is a colourless liquid with a characteristic sweet odour. It is highly reactive, though not with water, and may polymerise in the presence of oxygen, heat and light. Its vapours are both toxic and flammable. Aluminium alloys, copper, silver, mercury and magnesium are unsuitable for vinyl chloride service. Steels are, however, chemically compatible.
Ethylene oxide and propylene oxide are colourless liquids with an ether-like odour. They are flammable, toxic and highly reactive. Both polymerise, ethylene oxide more readily than propylene oxide, particularly in the presence of air or impurities. Both gases may react dangerously with ammonia. Cast iron, mercury, aluminium alloys, copper and alloys of copper, silver and its alloys, magnesium and some stainless steels are unsuitable for the handling of ethylene oxide. Mild steel and certain other stainless steels are suitable as materials of construction for both ethylene and propylene oxides.
Chlorine is a yellow liquid, which evolves a green vapour. It has a pungent and irritating odour. It is highly toxic but is non-flammable though it should be noted that chlorine can support combustion of other flammable materials in much the same way as oxygen. It is soluble in water forming a highly corrosive acid solution and can form dangerous reactions with all the other liquefied gases. In the moist condition, because of its corrosivity, it is difficult to contain. Dry chlorine is compatible with mild steel, stainless steel, monel and copper. Chlorine is very soluble in caustic soda solution, which can be used to absorb chlorine vapour.
Toxicity
Toxicity is the ability of a substance to cause damage to living tissue, impairment of central nervous system, illness or, in extreme cases, death when ingested, inhaled or absorbed through the skin. Exposure to toxic substances may result in one or more of the following effects.
- Irritation of the lungs and throat, of the eyes and sometimes of the skin. Where irritation occurs at comparatively low levels of exposure, it may serve as a warning which must always be obeyed. However, this cannot be relied upon since some substances have other toxic effects before causing appreciable irritation.
- Narcosis, which results in interference with or inhibition of normal responses and control. Sensations are blunted, movements become clumsy and reasoning is distorted. Prolonged and deep exposure to a narcotic may result in anaesthesia (loss of consciousness). While a victim removed from narcotic exposure will generally fully recover, the danger is that while under the influence he will not respond to normal stimuli and be oblivious of danger.
- Short or long term or even permanent damage to the body tissue or nervous system. With some chemicals this may occur at low levels of concentration if exposure is prolonged and frequent.
Threshold Limit Values (TLV)
As a guide to permissible vapour concentrations for prolonged exposure, such as might occur in plant operation, various governmental authorities publish systems of Threshold Limit Value (TLV) for the toxic substances most handled by industry. The most comprehensive and widely quoted system is that published by the American Conference of Governmental and Industrial Hygienists (ACGIH). The recommended TLVs are updated annually in the light of experience and increased knowledge.
The ACGIH system contains the following three categories of TLV in order adequately to describe the airborne concentrations to which it is believed that personnel may be exposed over a working life without adverse effects. TLV systems promulgated by advisory bodies in other countries are generally similar in structure.
- TLV-TWA. Time weighted average concentration for an 8-hour day or 40-hour week throughout working life.
- TLV-STEL. Short term exposure limit in terms of the maximum concentration allowable for a period of up to 15 minutes duration provided there are no more than 4 such excursions per day and at least 60 minutes between excursions.
- TLV-C. The ceiling concentration, which should not be exceeded even instantaneously. While most substances that are quoted are allocated a TLV-TWA and a TLV-STEL, only those which are predominantly fast-acting are given a TLV-C.
TLV are usually given in ppm (parts of vapour per million parts of contaminated air by volume) but may be quoted in mg/r& (milligrams of substance per cubic meter of air). Where a TLV is referred to but without the indications TWA, STEL or C, it is the TLV-TWA which is meant. However, TLV should not be regarded as sharp dividing lines between safe and hazardous concentrations and it must always be best practice to keep concentrations to a minimum regardless of the published TLV. TLVs are not fixed permanently but are subject to revision. The latest revision of these values should always be consulted. TLV presently quoted by ACGIH for some of the liquefied gases but it must be appreciated that the application of TLV to a specific work situation is a specialist matter.
Methods of liquefaction
Evaporation
A liquid change to gas is called evaporation. This may happen by evaporation or boiling. To achieve evaporation, heat of evaporation is needed. Some liquids evaporate very quickly, such as gasoline and ether. Other liquid substances evaporate very slowly, such as in crude oil. Evaporation is vapour formed out of the liquid surface and occurs at all temperatures.
This is explained by some of the liquid’s surface molecules being sent into the air, which is strongest at high temperatures, dry air and fresh wind. The specific temperature calls the amount of heat needed for one kilo of liquid with fixed temperature to form into one kilo of steam with the same temperature′′. The heat from evaporation is set free when the steam forms to liquid again, or condenses.
The heat necessary to evaporate one kilo of a certain liquid is called “specific heat of evaporation“, abbreviated as (r). The unit for specific heat of evaporation is J/kg.
Boiling
Boiling is steam formed internally in the liquid. The boiling occurs at a certain temperature, called “the boiling point“. Water is heated in normal atmospheric pressure (1 atm), in an open container. In common, some parts of air are always dissolved. The rise in temperature is read from a thermometer placed in the liquid’s surface. When the temperature has reached 100 °C, steam bubbles will form inside the liquid substance, especially in the bottom of the container. With continuous heat supply, the bubbling will rise like a stream towards the surface and further up into the air. The water is boiling.
The formation of bubbling steam can be explained as follows:
- During the heating, the water molecule’s kinetic energy increases, consequently the molecules demand more space. During the boiling, as long as there is water in the container, the temperature will be 100 °C.
- The boiling point is dependent upon the pressure. If the steam or the atmospheric pressure increases above liquid substance, the boiling point will also rise. If the surface temperature is just below the boiling temperature, then the water steam will evaporate on the surface. The evaporation point and the boiling point will be the same accordingly.
- The pressure from the surrounding liquid is the total amount of pressure above the liquid, Pa, plus the static liquid pressure.
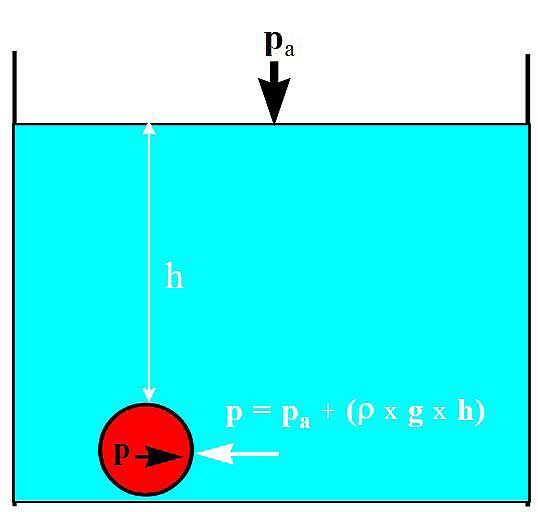
где:
- P = pressure in Pascal (100 000 Pa + 1 bar);
- Pa = barometer pressure;
- ρ = the liquid density in kg/m3;
- g = force of gravity acceleration (9,81 m/s2);
- h = liquid column in meter.
When reducing the pressure above the liquid, the boiling point will also be reduced. A practical use of this characteristic is the production of fresh water on board (fresh water generator).
Condensation
Condensation is the opposite of evaporation. If a gas is to be changed to liquid at the same temperature, we must remove the heat of evaporation from the gas. A gas can be condensed at all temperatures below the critical temperature. By cooling a gas, the molecule speed decreases hence the kinetic speed. The internal energy decreases, as well as, the molecule units and liquid forms.
Distillation
Distillation is a transferring of liquid to vapour, hence the following condensing of vapour to liquid. Substances, which were dissolved in the liquid, will remain as solid substance. With distillation it is possible to separate what has been dissolved from the substance, which was being dissolved. When a mixture of two liquids with different boiling point is heated, will the most volatile liquid evaporate first while the remaining becomes richer on the less volatile? On board, for instance, seawater is distillated by use of an evaporator.
Saturated, Unsaturated or Superheated Steam
Let us imagine boiling water, releasing vapour from a container, leading the steam into a cylinder that is equipped with a tightening piston, a manometer and two valves. The steam flows through the cylinder and passes the valves, whereon the valves are closing. There now is a limited and fixed volume of steam in the cylinder. Around this cylinder a heating element is fitted. Vapour from the container is constantly sent through this heating element to ensure that the temperature is maintained constant.
The piston is pressed inwards, and now the manometer should show a rise in pressure. But, the manometer shows an unchanged pressure regardless how much the volume is reduced. What’s happening is, the further the piston is pressed inwards, some parts of the steam is condensed more using less volume. The vapour from the heating element removes the condensed heat, which is liberated during the condensation process.
We find that the amount of steam, which is possible to contain per volume unit, remains constant when the steam’s temperature is equal to the condensation point at the set pressure. The room cannot absorb more vapour, it is saturated with steam and called “saturated“. If the piston is pressed outwards, the pressure will still show constant. The conclusion is:
- With temperature equal to the condensation point by set pressure, steam is saturated.
- Steam above boiling water is saturated.
- Saturated steam with a set temperature has a set pressure. This is called saturation pressure.
- With constant temperature saturated steam cannot be compressed.
- This also concerns vapour as saturated steam of other gases. Using the same cylinder arrangement as before.
The cylinder contains saturated steam, no water. The piston is drawn outward. When no water exists over the piston no new steam will be supplied underneath. The manometer will now show reduced (falling) pressure as the steam expands. When saturated steam expands without supplying new steam, it is called unsaturated steam. The room has capacity to collect more steam.
Unsaturated steam contains lower pressure than saturated steam at the same temperature. The unsaturated steam in the cylinder can be made saturated again in two ways. Either by pushing the piston inward to the originated position, or let the unsaturated steam be sufficiently cooled down. When the temperature is reduced, the saturation pressure will reduce. Unsaturated steam will, in other words, have a too high temperature to be saturated with the temperature it originally had. Therefore, this often is referred to as superheated steam.
Hazards from liquefied gas
This section deals with the properties common to all or most bulk liquefied gas cargoes. These cargoes are normally carried as boiling liquids and, as a consequence, readily give off vapour.
The common potential hazards and precautions are highlighted in the following sections.
Flammability
Almost all cargo vapours are flammable. When ignition occurs, it is not the liquid which burns but the evolved vapour. Different cargoes evolve different quantities of vapour, depending on their composition and temperature.
Flammable vapour can be ignited and will burn when mixed with air in certain proportions. If the ratio of vapour to air is either below or above specific limits the mixture will not burn. The limits are known as the lower and upper flammable limits, and are different for each cargo.
Combustion of vapour/air mixture results in a very considerable expansion of gases which, if constricted in an enclosed space, can raise pressure rapidly to the point of explosive rupture.
Toxicity
Some cargoes are toxic and can cause a temporary or permanent health hazard, such as irritation, tissue damage or impairment of faculties. Such hazards may result from skin or open-wound contact, inhalation or ingestion.
Contact with cargo liquid or vapour should be avoided. Protective clothing should be worn as necessary and breathing apparatus should be worn if there is a danger of inhaling toxic vapour. The toxic gas detection equipment provided should be used as necessary and should be properly maintained.
Asphyxia
Asphyxia occurs when the blood cannot take a sufficient supply of oxygen to the brain. A person affected may experience headache, dizziness and inability to concentrate, followed by loss of consciousness. In sufficient concentrations any vapour may cause asphyxiation, whether toxic or not. Asphyxiation can be avoided by the use of vapour and oxygen detection equipment and breathing apparatus as necessary.
Anaesthesia
Inhaling certain vapours (e.g. ethylene oxide) may cause loss of consciousness due to effects upon the nervous system. The unconscious person may react to sensory stimuli, but can only be roused with great difficulty.
Anaesthetic vapour hazards can be avoided by the use of cargo vapour detection equipment and breathing apparatus as necessary.
Frostbite
Many cargoes are either shipped at low temperatures or are at low temperatures during some stage of cargo operations. Direct contact with cold liquid or vapour or uninsulated pipes and equipment can cause cold burns or frostbite. Inhalation of cold vapour can permanently damage certain organs (e.g. lungs).
Ice or frost may build up on uninsulated equipment under certain ambient conditions and this may act as insulation. Under some conditions, however, little or no frost will form and in such cases contact can be particularly injurious.
Appropriate protective clothing should be worn to avoid frostbite, taking special care with drip trays on deck which may contain cargo liquid.
Comparison of hazards in liquefied gas carriage and in the transport of normal petroleum
While the carriage of liquefied gases incurs its own special hazards, some of its features are less hazardous than those of the heavier petroleum.
The following is a brief summary.
Hazards peculiar to carriage of liquefied gases:
- Cold from leaks and spillages can affect the strength and ductility of ship’s structural steel.
- Contact by personnel with the liquids, or escaping gases, or with cold pipework can produce frost burns.
- Rupture of a pressure system containing LPG could release a massive evolution of vapour.
Features of liquefied gas carriage resulting in a reduction of hazard compared with normal tanker operation:
- Loading or ballasting do not eject gases to atmosphere in vicinity of decks and superstructures. Gas-freeing is rarely performed and does not usually produce gas on deck.
- Liquefied gas compartments are never flammable throughout the cargo cycle. Static electricity and other in-tank ignition sources are therefore no hazard.
- There is no requirement for tank cleaning and its associated hazards.
