This article contains general info about LPG Gas Transportation, like Cargo Calculations, Safety, Fire detection, Fire-fighting and Basic Recommendations.
The main difference between making cargo calculations for a conventional tanker and for a gas tanker is that in the conventional tanker only the liquid product loaded or discharged is calculated. Thus, a tank empty of all liquid is regarded as empty. In a liquified gas tanker, however, because the liquid product is easily converted into a vapour, the quantity of vapour remaining on board after discharge (or converted into a liquid during loading) must be taken into account, in addition to the liquid loaded or discharged, when making the cargo calculations.
- Cargo calculations
- Making the Calculations
- To Calculate the Quantity of Liquid on Board (Metric)
- To Calculate the Weight of Vapour on Board (Metric and Imperial)
- Assessing the volume occupied by the vapour
- To Calculate the Quantity of Liquid on Board (Imperial)
- To Calculate the Correct Volume of Liquid to Load when Loading a Full Cargo
- Semi-refrigerated and Pressure Ships
- Expansion Relief Valves on Liquid Pipelines
- To Calculate the Correct Volume to Load when Taking a Part Cargo
- To Calculate the S.V.P, of a Mixture of Products at a Given Temperature
- To Calculate the Individual Proportions of Vapour in the Vapour above a Liquid Mixture
- Molecular Weights
- Aid to Memorising the Formulae
- Comparison of Metric and Imperial Systems
- Other Points to be Borne in Mind
- Safety
- Prevention
- Safe Navigation
- Safe Practice
- Gas Detection
- Detection
- Fire Detection
- Fighting
- Fire-fighting
- Oxygen-deficiency
- Precautions to be Taken when Entering Spaces which May Have a Deficiency of Oxygen
- Recommendations
- Safe Navigation
- Harbour Control
- Enforcement of Traffic Separation Systems
- Emergency Isolation Valves for Safety Valves
- Greater Consultation between Operators and Design Staff
- Glossary of terms used
- Gas laws
- Heat
- Pressure
- Temperature
Cargo calculations
Because the cargo tanks of practically all liquified gas tankers are calibrated in cubic metres, this will be assumed to be the case in the examples which follow. If the cargo tanks are calibrated in units other than cubic metres, the principles of calculation remain the same, but the necessary conversion factors will have to be used. The procedure for making the calculations is as follows.
Prior to loading, the shore terminal should provide the vessel with the density at 15 °C. of the product to be loaded. In this connection, it is extremely rare for a vessel to load a pure product (except in the case of ammonia). Commercial propane usually contains small proportions of other hydro-carbons, butane, ethane, etc., which have the effect of slightly altering the density of the product (and with butane, also). On completion of loading, the terminal authority usually provides the vessel with a chemical analysis of the product loaded.
To ascertain the quantity loaded, the small quantity of product on board prior to loading (heel plus weight of vapour) is subtracted from the total quantity of product on board after loading. In the case of ascertaining the quantity of cargo discharged, the quantity of product remaining on board after discharge is subtracted from the total quantity of product on board prior to discharge.
Making the Calculations
After the tank readings (tank pressure, liquid and vapour temperatures and the tank soundings—depths of liquid) have been agreed with the shore representatives, various adjustments have to be made before the volume occupied by the product can be correctly assessed. The adjustments are:
- Trim correction to the soundings as read, to make allowance for the fact that, in most cases, the tank liquid level indicating devices are not centrally located. This trim correction, when applied, gives the corrected sounding;
- With the corrected sounding, the volume of liquid is read from the tank calibration tables and, by subtracting it from the total volume of the tank, the vapour volume can be ascertained;
- Because the tank will have contracted if the tank temperature is below 15 °C., the volumes obtained from the calibration tables have to be multiplied by the shrinkage factor for the temperature concerned to give the corrected volume of the spaces occupied by the liquid and the vapour;
- Low-sounding trim corrections are also tabulated to allow for the wedge-shaped volume when the liquid level touches the forward end of the tank bottom.
To Calculate the Quantity of Liquid on Board (Metric)
Two methods can be used. The most direct method is to convert the density of the liquid at 15 °C. to its density at loading temperature and then to multiply the density at loading temperature by the corrected volume occupied by the liquid. The result will be in metric tons. To convert the density of the liquid at 15 °C. to its density at loading temperature, one can either consult the Density Reduction to 15 °C. Table (ASTM-IP Table 53) and obtain the density at loading temperature directly or, by consulting the Volume Reduction to 15 °C. (ASTM-IP Table 54), obtain the factor for the product at the loading temperature and then multiply the density at 15 °C. by the factor so obtained. Both methods should give the same answer.
Example:
- Density of product at – 15 °C = 0.509 kilogrammes per litre;
- Temperature of product on board –39 °C;
- Sounding as read 12 metres – 32 cms;
- Trim correction – 7 cms;
- Corrected sounding 12 metres – 25 cms;
- Liquid Volume – 4567.890 m3;
- Shrinkage factor – (-39 °C.) 0.9988;
- Corrected Volume – 4562.409 m3;
- Density of product at – 39 °C. obtained directly from Table 53 = 0.579 kilogrammes per litre;
- Volume Reduction factor for product at -39 °C. obtained from Table 54 is 1.137 : 0.509 × 1.137 = 0.578733;
- Corrected Volume 4562.409 × 0.579 =2641.348 metric tons 4562.409 × 0.578733 = 2640.417 metric tons.
The second method is to adjust the corrected volume occupied by the liquid at the loading temperature so that it is equivalent to the volume it would occupy if the liquid were allowed to warm up and expand to 15 °C. This is done by multiplying the corrected volume by the coefficient of expansion for the temperature concerned, as shown in the Volume Reduction to 15 °C. Table (ASTM-IP Table 54). The volume occupied at 15 °C. is then multiplied by the density at 15 °C. to give the quantity in metric tons. Working the same example shown above, commencing at the corrected Volume, we get:
- Corrected Volume – 4562.409 m3;
- Volume reduction Factor – 1.137;
- Volume at – 15 °C. 5187.459 m3;
- Volume at – 15 °C. 5187.459 x 0.509 =2640.417 metric tons.
Both methods give the same result (or very nearly so) because the same factor (Volume Reduction) can be used either to obtain the density at loading temperature or to obtain the volume the liquid would occupy at 15 °C. In other words, the three factors used are the same, namely, volume at loading temperature, Volume Reduction Factor and density at 15 °C.
The second method is preferred to the first for the following reasons:
- Product is frequently sold by the litre and 15 °C. is a more practical temperature for the distributor than, say, — 40 °C;
- A table of Volume Reduction Factors will cover a fairly wide range of densities, whereas to list each density separately with the same degree of accuracy requires a much larger set of tables. Due to the restriction of the number of decimal places given in the density reduction table, small arithmetical differences arise.
To Calculate the Weight of Vapour on Board (Metric and Imperial)
The vapour calculation is quite simple and the formula is based on the following laws:
- Gay Lussac’s Law: The density of a gas at standard temperature and pressure is proportional to its molecular weight;
- Charles’s Law: The volume of a given mass of gas is directly proportional to the absolute temperature, provided the pressure remains constant. If the formula is transposed, it can be deduced that the density of a given mass of gas varies inversely with the absolute temperature provided the pressure remains constant;
- Boyle’s Law: The volume of a given mass of gas is inversely proportional to its pressure, provided the temperature remains constant (or density varies with the absolute pressure, provided the temperature remains constant);
- 1/22400 density of hydrogen H2 which gives the theoretical density of 1 cubic metre of H (as opposed to H2) — the simplest and lightest atom. (See section relating to molecular weights.).
Written for a fixed temperature, the individual effects of these laws are:
- Gay Lussac’s Law = Density varies with molecular weight of the vapour;
- Charles’s Law = Density varies inversely with absolute temperature;
- Boyle’s Law = Density varies with absolute pressure;
- 1/22 400 = A constant.
The conclusions derived from the three laws can be combined to give the formula for Vapour Wt. as:
Assessing the volume occupied by the vapour
The vapour volume is obtained by:
- subtracting the volume of liquid in each tank from the total volume of each tank;
- the separate vapour volumes of each tank are then multiplied by the shrinkage factor to give the corrected vapour volume for each tank;
- the corrected vapour volumes for each tank are then added together to give the total corrected vapour volume.
The total corrected vapour volume is then used with the mean pressure and temperature to make the vapour calculation.
Example:
What is the weight of 29,952 cubic metres of propane vapour at 0.1 bars gauge pressure at — 30 °C.?
0.1 bars gauge pressure = 1.1 bars pressure absolute — 30 °C. = 243 absolute temperature.
Molecular weight of propane = 44 (See Table at end of Chapter).
Another method is to calculate the weight of air corresponding to the pressure and volume required and then:
Because air is roughly a mixture of 80 per cent. nitrogen and 20 per cent. oxygen and
Taking air as having a density of 0.001293 metric tons per cubic metre at 0 °C., using the formula provided by Charles’s Law,
a table of densities for each degree centigrade can be compiled. Re-working the same example given above, using the short method, we get:
The answers are not exactly the same, but they are sufficiently close to bear comparison.
To Calculate the Quantity of Liquid on Board (Imperial)
The method used is basically the same as that for the metric system except that barrels or cubic feet are used as a measurement of volume and temperature is measured using the Fahrenheit scale, and the Specific Gravity at 60 °F., is given instead of density.
The steps are:
- converting the corrected volume in cubic metres to barrels (or cubic feet);
- using the volume reduction to 60 °F. factor (ASTM-IP Table 24), converting the volume in barrels into that which the liquid would occupy at 60 °F., giving net barrels;
- consulting the table which gives long tons per barrel for various Specific Gravities (ASTM-IP Table 29), obtaining the required factor and multiplying the net barrels by this factor. The answer will be in English long tons.
To Calculate the Correct Volume of Liquid to Load when Loading a Full Cargo
The maximum volume of cargo that may be loaded into a cargo tank is governed by the relief pressure setting on the cargo tank safety valves. The rule is that no more cargo should be taken than that which would occupy 98 per cent. of the cargo space after allowance has been made for the cargo to expand to a temperature, the saturated vapour pressure of which would lift the safety valve. In effect, this means that if for any reason it is not possible to refrigerate the cargo, the cargo will expand as it warms up and, at the same time, its vapour pressure will rise, until at a certain temperature, the increase in vapour pressure will cause the safety valves to open and the excess pressure relieved up the mast. The release of the excess pressure will cause the product inside the tank to boil, use up latent heat and, in this manner, the cargo to refrigerate itself so that it will not get any warmer. The ship must be so loaded that when the temperature/pressure of the cargo corresponds to the safety valve relief setting, the cargo occupies 98 per cent. of the cargo space.
The calculation is made in the following stages:
- From a table or graph, ascertain the temperature at which the saturated vapour pressure of the product corresponds with the relief setting of the safety valve;
- Ascertain the density (or specific gravity) of the product at:
- the temperature at which the saturated vapour pressure of the product corresponds with the safety valve setting;
- the density (or specific gravity) of the product at the loading temperature.
- Dividing the density at “lift-off” temperature by the density of the product at loading temperature and multiplying by 98 gives the percentage of the volume which can be loaded;
- Multiply the percentage so obtained by the total volume of the tank and this will give the maximum volume that can be loaded in that tank.
From the calibration tables, the sounding corresponding to this volume can be ascertained.
Semi-refrigerated and Pressure Ships
The coefficient of expansion for liquid gases is very high and in semi-refrigerated ships the allowance for expansion can be very high. Where the rise in saturated vapour pressure due to temperature increase is such as to be unlikely to equal the safety valve setting (e.g. butane), the cargo tanks are so filled that the tanks are 98 per cent. full when the cargo temperature reaches 45 °C., the highest temperature likely to be reached in service. Vessels capable of carrying both semi-refrigerated cargoes and fully refrigerated cargoes at atmospheric pressure usually have the facility of being able to alter their safety relief valve settings from about 1.7 bars when carrying atmospheric cargoes to about 6.3 bars when carrying semi-refrigerated cargoes, by the superimposition of stronger springs on the safety valves.
In pressure ships which do not refrigerate their cargoes, all cargoes are so loaded as to occupy 98 per cent. of the cargo tank volume when the temperature reaches 45 °C. In practice this usually works out at about 85 to 90 per cent. of the cargo tank capacity at normal temperatures, but the exact percentage of the volume to fill must always be worked out.
Expansion Relief Valves on Liquid Pipelines
Due to the high coefficient of expansion of the products carried, all liquid lines capable of isolation (i.e. those sections of liquid lines between shut-off valves) are provided with safety relief valves to prevent the lines concerned splitting due to the “hydraulic effect” occasioned by expansion of the liquid inside the pipeline.
In this connection, the ship’s pipelines are usually rated ASA — 150 or ASA — 300, the figures 150 and 300 relating to the safe working pressure of 150 p.s.i. and 300 p.s.i., respectively. The manifold connections are such that an ASA — 150 connection will not fit an ASA — 300 connection without the use of a special adaptor, so that those responsible for the conduct of cargo operations are made aware of the pressure restriction and can be careful not to overstress the pipelines.
To Calculate the Correct Volume to Load when Taking a Part Cargo
In this case, allowance must be made for the weight of the vapour displaced by the liquid loaded.
The calculation is made in the following way:
- Divide the metric tons to be loaded by the density of the product at the loading temperature. The result will be approximately the volume in cubic metres of liquid to load;
- Ascertain the weight of vapour displaced by the liquid (weight of vapour of the volume obtained in (a), above) and increase the original quantity of liquid to load by this amount.
To Calculate the S.V.P, of a Mixture of Products at a Given Temperature
It is sometimes necessary to work this out when carrying mixtures of products. The calculation is made in four stages, namely:
- Divide the component weights of the mixture by their respective molecular weights;
- Add the results together and then divide each individual result by the sum of all the results. This gives the mol fraction;
- Multiply the SVP, of each product at the temperature concerned by its mol fraction. This gives the partial pressure exerted by each product;
- Add the partial pressures and, by Dalton’s Law of Partial Pressures, the sum of the partial pressures is the total absolute saturated vapour pressure exerted by the mixture.
Example:
What is the SVP, of a mixture of 10 tons of propane and 10 tons of butane at +10 °C.? SVP Propane at +10 ° C. = 6.32 Bars absolute: Molecular Wt. = 44 SVP Butane at + 10 °C. = 1-49 Bars absolute: Molecular Wt. = 58
- ;
- ;
- Partial pressure exerted by propane = 0.5687 x 6.32 = 3.59. Partial pressure exerted by butane = 0.4313 x 1.49 = 0.64;
- Total SVP of mixture = 4.23 bars absolute or 3.23 bars gauge.
To Calculate the Individual Proportions of Vapour in the Vapour above a Liquid Mixture
To do this, the total pressure above the mixture should be divided by the partial pressures exerted by each product and the results will be the individual proportions of vapour in the vapour above the mixture.
In the example given above, the individual proportions are:
- Propane 3.59/4.23 = 0.85 or 85 per cent;
- Butane 0.64/4.23 = 0.15 or 15 per cent.
NOTE: Mixtures are usually by volume, not by weight, in which case the respective weights constituting the mixture must be determined before making the above calculations.
Molecular Weights
Because frequent reference is made to molecular weights, a brief reference to them and how they are determined is given below.
The molecular weight of a molecule is the sum of the atomic weights of the elemental atoms which comprise the molecule. In this connection, a molecule consists of at least two atoms, so elemental molecules consist of two elemental atoms. Thus, the chemical formula for hydrogen is written H^ oxygen 0^ nitrogen N^ etc. Some useful atomic weights are listed below.
- Hydrogen = 1;
- Carbon = 12;
- Nitrogen = 14;
- Oxygen = 16;
- Chlorine = 35.
NOTE: These figures are not exactly correct because the weight of the electrons has been disregarded, but they are very nearly correct and are sufficiently accurate for our purpose. The chemical formulae of most of the products carried are listed below, together with their molecular weights.
Aid to Memorising the Formulae
The names of the saturated hydro-carbons end in “ane”. They are stable compounds and have no tendency to change their state, and are normally burnt as fuel. In the order above (which is the order of their respective atmospheric boiling temperatures), methane has one carbon atom, ethane 2, propane 3, etc. If the number of carbon atoms is doubled and 2 added, that is the number of hydrogen atoms in the saturated product.
The unsaturated products have the same number of carbon atoms as their saturated equivalents, but two hydrogen atoms have been removed from the molecule, so that ethane becomes ethylene, propane becomes propylene, butane becomes butylene. The removal of two hydrogen atoms makes the molecules comprising the product chemically unstable. The molecules tend to link up with one another to form very long molecules, particularly in the presence of oxygen, forming polymers. The process of forming polymers is called polymerisation. This must be avoided, so unsaturated products are loaded into an oxygen-free environment.
Butadiene is a doubly unsaturated product with 4 hydrogen atoms removed from the butane molecule. It is even more chemically unstable than butylene and, in the case of butadiene, an inhibitor is added to retard further the polymerisation process.
Iso-butane is an isotope of butane where the shape of the butane molecule has been altered, giving iso-butane somewhat different thermo-dynamic properties from those of butane, which is sometimes called normal butane, or n-butane.
Comparison of Metric and Imperial Systems
Fundamental differences exist between the two systems and an understanding of these differences is necessary when comparing the results of the two. The metric system is an absolute system, completely decimalised. The system is described as absolute because the weights are “in vacuo” weights (as weighed in a vacuum), whereas under the imperial system, the weights are “in air” weights, so that when comparing the weights, allowance has to be made for the weight of air displaced.
In the metric system, water has a density of 1 gramme per cubic centimetre at 4 °C. (39 °F.), when it is at its most dense. At 15 °C., its density would be somewhat less. For this reason, because weights of cargoes are calculated for a temperature of 15 °C. and not 4 °C., specific gravities are not used but the density of the product at 15 °C. used instead, so preserving the decimal virtues of the metric system.
Under the imperial system, the unit of weight is the pound and the unit of volume the gallon, which is that amount of space occupied by 10 pounds of water at 62 °F. when weighed in air. The complication does not end there because, in the oil industry, specific gravities are given at 60/60 °F. (i.e. the density of the product at 60 °F. compared with the density of water at 60 °F.). At 60 °F., water weighs very slightly more than 10 pounds per gallon and somewhat less than 1 gramme per cubic centimetre. For converting density at 15 °C. to S.G. 60/60 °F., ASTM-IP Table 51 should be consulted (and vice versa).
In order to compare the results obtained by the two systems, metric tons are converted to metric tons in air by using the factors provided in ASTM-IP Table 56. The metric tons can then be converted into long tons by multiplying by the factor 0.98421.
When making vapour calculations, the imperial system neglects the “in air” factor and makes use of the same method of calculation as does the metric system.
The foregoing explanation is given because discrepancies sometimes arise due solely to the different methods of calculation, combined with neglecting to use the factors mentioned above, particularly when trading between a country using the metric system and one using the imperial.
Other Points to be Borne in Mind
When carrying fully refrigerated cargoes, some receivers require very low “on arrival” tank pressures. If mercurial “U” tubes are used as tank pressure measuring devices, they are affected by fluctuations in the atmospheric pressure, whilst the pressures in the tank are absolute. Thus. a pressure of 0.04 bars at 1 010 millibars atmospheric pressure will show 0.02 bars at 1 030 millibars and 0.06 at 990 millibars.
If the cargo contains a relatively high ethane content, some loss in transit and difficulties with reliquifaction can be expected. Thus, a 2 per cent. by weight ethane content dissolved into the cargo will create about 18 per cent. ethane content in the vapour space.
Notes:
- 1Ethene, Propene and Butene are alternative names for Ethylene, Propylene and Butylene, respectively;
- 2Boiling temperatures are given for atmospheric pressure;
- 3SVPs listed are absolute pressures.
Some useful data:
- Standard (sea level) atmospheric pressure = 29.92 inches mercury, which is equivalent to 760 millimetres, or 1013.2 millibars. In vapour calculations, it is accepted practice to regard 1 bar (1 000 millibars) as 1 atmosphere, but this is not strictly correct.
Factors for converting:
- Metric tons (in air) to long tons = 0.98421;
- Long tons to metric tons = 1.01605;
- Cubic metres to barrels = 6.2898.
Density of air at standard atmospheric pressure and at 0 °C. = 0.001293 metric tons per cubic metre. Long tons NIL.
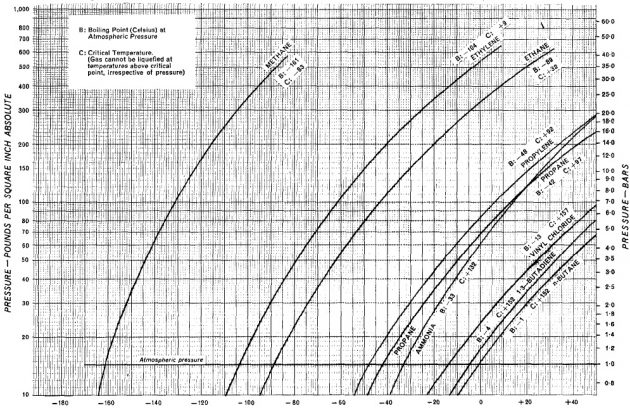
Temperature—degrees celsius:
- *Curves constructed by application of Antoine equation, using data from “Selected Values of Properties of Hydrocarbons and Related Compounds”, American Petroleum Institute Research Project 44, Thermodynamics Research Centre, Texas A. & M. University, and from “Advances in Chemistry Series:No. 22” (1959) published by the American Chemical Society.
Safety
The two main safety Properties and hazards of shipping LNG, LPGhazards are those of fire and the avoidance of entering oxygen-deficient spaces.
Fire, however caused, represents the biggest hazard to a liquified gas tanker. It is best examined from three distinct aspects:
- fire prevention;
- fire detection, so that in the event of a fire breaking out, it can be tackled in its early stages;
- fire fighting.
Prevention
This involves three main considerations:
- collision avoidance (safe navigation);
- safe practice;
- efficient detection of gas concentration before it reaches a flammable mixture.
Safe Navigation
The principal risk of collision will always lie in the long river or canal transits to the gas terminals, but those responsible for the navigation of the vessel can ensure that the transits are only made under favourable conditions, namely:
- good visibility (at least 1 mile), and;
- the Crew Evaluation Test online about Operation of Steering Gearsteering capability and engine reliability are perfect.
One of the biggest risks is the possible loss of all electric power (blackout). In consultation with the Chief Engineer, the electrical load and the supply available should be adjusted to reduce this risk to an absolute minimum. (It was an electrical supply failure that caused the Esso Maracaibo to collide with the Maracaibo bridge, causing it to collapse, resulting in considerable loss of life and extremely extensive damage.) In shallow water transits, where the vessel is required to make the transit at even keel. a reliable trim indicator must. be provided and consulted to ensure that the steering qualities of the ship are not adversely affected by the vessel trimming by the head due to shallow water effect.
Although the personnel of a gas tanker may take all the necessary navigational precautions, there is nothing they can do with regard to the operation of other vessels making the transit at the same time.
Safe Practice
This covers the correct handling of the products and ensuring that all the safety devices of the cargo handling machinery operate efficiently. For this reason, they should be regularly tested. Gas-freeing must be suspended when lightning is in the vicinity.
Read also: Safety Precautions and Measures on Gas tankers
Additionally, a constant watch must be kept for any leaky joints or glands and these repaired as soon as found. The most vulnerable spot for corrosion is the condensate line, which is the most used line. The cold product causes water vapour in the air in contact with the exposed portions of the line to condense and form frost or dew, which in time causes rusting. In practice, it has been found that the most likely danger points are where the insulation ends and a few inches back under the insulation. The pipes always rust from the outside inwards and, almost without exception, never internally. Pipes covered by saturated insulation are always suspect.
Gas Detection
This is of vital importance because it gives warning of a potential fire risk before the risk has reached dangerous proportions, in time for remedial action to be taken. The gas detector continuously monitors all spaces adjacent to the cargo, both in storage and handling, the most important being containment spaces, compressor room, motor room and instrument spaces. The detector is fitted in a cabinet from which sample lines lead to the various spaces to be tested, each sample line terminating in what amounts to a large valve chest in the gas detector. Each valve is held closed by a spring and opened by a solenoid when the line is being used for sampling. In the event of a concentration of gas reaching 30 per cent. of the explosive level, an alarm is sounded, and a light indicates the space affected.
There are two basic types of gas detectors, one operating on the principle of the heat of combustion varying the value of a resistance on a wheatstone bridge, so putting it out of balance and causing a current to flow across the bridge, measured by the percentage indicating device. The other type acts on the principle of infra-red absorption. The infra-red type of gas detector is being used in increasing numbers.
It is of vital importance that the detector should never be switched off and be kept running efficiently. An officer should be given the personal responsibility of keeping it in good order and ensuring that adequate supplies of span and zero gas are held on board for calibration purposes and that regular adjustments and calibrations are carried out in accordance with the manufacturer’s recommendations.
Detection
Fire Detection
Early fire detection is of the greatest importance so that a fire can be tackled in its early stages and before it gets a firm hold.
Most gas tankers are comprehensively fitted with fire detectors, which are of 3 basic types:
- heat sensors;
- combustion (smoke) detectors;
- flame detectors.
Heat detectors are the simplest type and are fitted in all accommodation and store spaces. They are actuated by the rise in temperature occasioned by fire.
Combustion detectors are fitted in all machinery spaces and compressor and motor rooms. They work on the principle that a fire emits particles of matter into the atmosphere. The majority are invisible to the naked eye: the remainder can be seen as smoke. The combustion detectors are sensitive to those particles invisible to the naked eye.
Flame detectors are fitted in the engine room. These detectors are sensitive to infra-red heat radiated from flames and are responsive to flame flickering. Because this form of detection can be masked by smoke; both types of detectors (flame and combustion) are fitted.
When any of these detectors are actuated, they sound a distinctive alarm and the location of the fire is indicated on a panel (or panels) suitably located.
Fighting
Fire-fighting
Most operators of LPG tankers, being seamen, are familiar with the procedure of combating fires in the accommodation and engine room, so this section is confined to fighting LPG fires.
Experiments ashore have shown that the most effective way of fighting LPG fires is to shut off the supply at source and extinguish the fire by starvation rather than by attempting to extinguish it by other means. This is because, if the fire were to be extinguished without stopping the supply of inflammable vapour, there is a very real risk of a cloud of vapour forming and suddenly re-igniting.
The main safety device is the emergency shut-down system which, when operated, shuts all valves in the cargo system and also shuts off the electrical current to the cargo handling equipment. The emergency shut-down system has fusible plugs incorporated in it, which, when heated (as by a fire), melt and operate the emergency shut-down system automatically.
Two sorts of LPG fires can be envisaged. The one due to ignition of an escape of liquid and/or vapour whilst the cargo system is intact, the other caused by a collision, when a cargo tank is ruptured.
In the first case, control of the emission of liquid or vapour can normally be established and the fire fought in accordance with the principles laid down, which is to shut off the source of supply of fuel and allow the fire to burn itself out, at the same time carrying out what amounts to extensive boundary cooling to prevent the fire from spreading. This involves turning on the bridge front sprays and, if possible, so to manoeuvre the ship that the flames blow clear.
Since a pool of LPG liquid will vaporise and burn all in one place, it should be possible for men wearing fire-proof suits approaching from upwind to work close to the fire, particularly if others further off keep them soaked with water. In this way a valve which may have failed to close automatically may be shut.
The effect of the fire may be to warm up the cargo in the other tanks, causing the safety valves to lift and add more fuel to the fire, so, in addition to cooling the area covered by the flames, all the tank domes and pipelines of the unaffected tanks must be cooled by solid jets of water. If any of the water turns to steam, that area requires additional cooling.
Flames from safety valves should never be extinguished; they are sufficiently far removed from a cargo tank as not to heat it. The vapour released should be allowed to burn itself out.
In the case of a fire resulting from a collision where a tank has been ruptured, there is no possible means of controlling the escape of fuel so as to extinguish the fire by starvation. The best chance of survival is to remove the ship from the area of spillage by going astern and bringing the stern up-wind so that the flames are blown clear over the bows. Control of the ship moving astern can be maintained by short bursts of speed ahead. It is useless to put the wind on the beam so that the flames are blown clear, because the leeway will cause the ship to drift into the middle of the pool of evaporating and burning liquid.
However, never is the adage “prevention is better than cure” more true than in relation to fire on board liquid gas vessels.
Oxygen-deficiency
Precautions to be Taken when Entering Spaces which May Have a Deficiency of Oxygen
The most likely spaces to have a deficiency of oxygen are spaces which were previously inerted and which may have been inadequately ventilated and ballast tanks which have been empty for some time in which rusting has taken place.
To understand the dangers of entering these spaces, a description of the effects of oxygen-deficiency and carbon dioxide excess is given.
Air consists basically of 79 per cent. nitrogen and 21 per cent. oxygen. Oxygen is the life-sustaining component and nitrogen acts as a dilutant. When breathing, part of the oxygen content is turned into carbon dioxide, whilst the nitrogen content remains unchanged.
Carbon dioxide stimulates the breathing; too much overstimulates it, causing the individual to pant and in excessive quantities (over 10 per cent.) it is a poison and kills.
In normal circumstances (e.g. an unventilated room), as oxygen is used up, it is replaced by carbon dioxide and eventually those inside will “gasp for air” and be uncomfortable, but it is the overstimulation of the breathing due to an excess of carbon dioxide being present that causes the discomfort, not the lack of oxygen. If the carbon dioxide content of the atmosphere were removed, all signs of discomfort would disappear, but all inside would suffer from a lack of oxygen.
It is the complete lack of discomfort experienced with oxygen-deficiency that is the biggest danger. Oxygen-deficiency affects first the brain, causing the victim to become, in turn, befuddled, stupid and sleepy, eventually to die if the oxygen content falls below 10 per cent.
This information is provided to warn operators of the special dangers to be met when entering the spaces referred to and of the very thorough ventilation required. In the case of ballast tanks, these should be completely filled with water and then emptied before entry.
In the case of containment spaces, ventilation must be thorough and prolonged. Those working in the containment space must have a ventilation chute of the concertina type (elephant’s trunk) with them, air being supplied by an air-driven fan on deck close to where they are working.
When in spaces adjacent to inerted containment spaces, (e.g. in a double bottom under a containment space), the pressure in the inerted space should be reduced to zero to exclude the possibility of inert gas leaking into the double bottom tank.
Rescue breathing apparatus should be placed at the entrance to the tank or containment space, with a man to maintain contact with the working party, usually by portable radio.
Recommendations
This section is controversial and is intended to promote thought and discussion. Its object is to point out directions along which improvements can be made without waiting for recommendations following a post mortem after a disaster has taken place.
Safe Navigation
Operators of liquified gas tankers must be among the most safety-conscious seamen in the world. First and foremost, collision is considered to be by far the greatest hazard. The effects of a collision causing the rupture of a cargo tank would be extremely serious, but fortunately such an event has not yet occurred, not even in the collision between the Yuyo Maru and Pacific Arest Japanese Maritime Safety Agency Report, dated March, 1975.x. It is possible, however, to visualise the effects. If a tank of 10,000 cubic metres were ruptured, approximately 6,000 tons of propane would be affected. That proportion which spilled into the sea would vaporise very quickly, but although the bulk of the vapour cloud so formed would be over-rich and not burn, that part of it in contact with air and not over-rich would almost certainly be ignited by the sparks and heat generated by the collision and burn around the edges with great intensity, drawing in more air to sustain combustion. The heat of this combustion, combined with the heating effect of any water that entered the ruptured tank, would cause the remainder of the product left inside the tank to vaporise so that practically all 6,000 tons would be burnt. In short, it would be equivalent to a multi-thousand bomber raid in World War II. It is quite likely that other of the ship’s cargo tanks would be affected, magnifying the effect.
That is the hazard to be avoided, and it really is not much use hoping that it will never happen. Some positive preventing action is needed.
In the long term, it would be a great advantage if long river or canal transits were reduced by placing gas terminals near the mouth of a river in areas of low population density. This would reduce the risk of collision by shortening the confined water transit and reduce the possible disastrous environmental effects of a collision should one take place.
Harbour Control
A great deal can be done to increase safety through harbour control. The United States and Japanese governments have given the world a lead in laying down rules for safe navigation when using their ports. The rules are tailored to suit individual ports, but cover:
- Minimum visibility for making the entry. Under Japanese regulations, entering and leaving must be carried out in daylight only in good visibility. U.S. regulations restrict the movement of loaded vessels to daylight in good visibility. Empty vessels may move at night, provided the visibility is adequate;
- Provision of escort which proceeds ahead of the liquified gas tanker to enforce traffic regulations;
- In some cases the movement of other ships is restricted by harbour control services whilst a gas tanker is moving.
Additionally, the U.S. and Japanese governments inspect gas tankers to satisfy themselves that the ships are in efficient working order and well maintained. In fact, a U.S. Coastguard Certificate of Compliance (with their requirements) has come to be regarded by many authorities outside the U.S. as a guarantee of efficiency.
Enforcement of Traffic Separation Systems
Although international traffic separation systems have been in existence for some time, little has been done beyond moral persuasion to enforce them. Such a lax situation would never be tolerated on the roads and, by way of a start, wilful breaking of traffic separation rules should void the insurance, in the same way as an unjustified deviation does.
Emergency Isolation Valves for Safety Valves
These should be fitted because, although the safety valves on liquified gas carriers are generally very reliable, they have occasionally operated below their correct pressure settings and also failed to re-seat properly after having operated.
There is a hazard if, due to a malfunction of a safety valve, large quantities of LPG or ammonia vapour are released unnecessarily into the atmosphere, with no means of stopping the release.
The isolation valves would be linked together so that only one of a pair of safety valves could be isolated at any one time, leaving the other safety valve still in service. Under operating conditions, both isolating valves would be in the open position so that both safety valves are in service.
If a nitrogen line were fitted between the isolation and safety valves, an additional facility would be provided for the regular testing of each safety valve. Without an isolation valve, the only practicable means of testing and adjusting the safety valves is to pressurise the whole cargo tank. As the ship is rarely gas-free, this involves the release of a substantial quantity of vapour into the atmosphere and this can only be done at sea. It is very difficult and expensive to arrange for the “Classification Society” surveyors to supervise the operation and then put their seal on the tested valves.
When a liquid gas tanker arrives in port, particularly a loading port, the harbour heads, which increase the relief setting of the safety valves, should be fitted to prevent the undesirable release of vapour when the cargo tanks are being cooled by the process of the evaporation of liquid product whilst it is being sprayed into the tanks; If, however, surveyors’ seals have been placed on the safety valves, these harbour heads cannot be fitted without disturbing the seals. Were isolation valves fitted, these would permit the testing of the safety valves before the cargo operation commenced and could then be sealed in the open position.
Although superficially increasing the relief pressure setting of the safety valves may appear to increase the risk of straining the cargo tank by over-pressurising it, this is not the case. The cargo tanks being empty, without the weight of liquid in them, no extra strain is involved except at the top of the tank. A ship which has a normal safety relief setting of 0.3 bar must work close to this setting in order to cool down. Increasing the relief setting to 0.4 bar does not imply that the operators would increase the pressure in the tank to this level. They would still work to the normal pressures, but if the pressure inadvertently increased, say, to 0.32 bar, no vapour would be released. The environment would be much protected because the risk of vapour release is reduced.
The settings of the safety valves may alter slightly during the course of a year, but the fitting of isolation valves would overcome this difficulty by permitting tests, adjustments and, if necessary, repairs to the valves to be made at frequent intervals and so provide greater security than annual tests and surveyors’ seals.
Greater Consultation between Operators and Design Staff
There must be greater liaison between those who operate the ships and those who design them. The main areas of bad design relate to:
- Unsuitable valves. Frequently it would appear that little thought is given to the function of a valve and the type of valve provided to fulfil a particular function;
- Inefficient lay-out of piping systems;
- Bad lay-out of monitoring equipment.
Operators are in a good position to give advice because their experience covers ships designed by a great number of firms in different countries, and having, operated the ships, are in a position to appreciate the good points for inclusion in future construction.
Finally, seamen should be consulted before drastic changes are made. The replacement of hydraulic steering control by electrical control is a good example. Hydraulic control enables a quartermaster to steer the ship without having constantly to watch the helm indicator, because he knows when the wheel is amidships and that a spoke or two either way enables him to steer the ship.
This is not so with the usual form of electrical control, the rudder moving when a contact is made and stopping when the contact is broken. To bring the rudder back to amidships, the contact must be made in the opposite direction and stopped when the rudder indicates amidships. This means that the quartermaster must continuously refer to the helm indicator and take his eyes off the compass or steering mark to do so. No motor manufacturer who wished to provide his vehicles with power-assisted steering would be allowed to market such a system.
Electrical control is ideal for automatic steering when the ship is at sea, but requires considerable skill to operate manually and with the ship being steered automatically at sea, many seamen get little practice in its use. The views of pilots would be very valuable in this regard.
Glossary of terms used
BOILING: This is the action which takes place when a liquid changes its state from a liquid into a gas or vapour. The heat required to bring this change of state about is called Latent Heat.
BOILING TEMPERATURE: This is the temperature at which a liquid boils. As the boiling temperature rises with an increase in pressure (see saturated vapour pressure), the boiling temperatures are usually given for atmospheric pressure. At this pressure, water boils at +100 °C., butane at – 0.5 °C., ammonia at – 33 °C. and propane at – 43 °C.
CONDENSATION: This is evaporation in reverse. If a vapour becomes supersaturated, condensation takes place and heat is surrendered. For example, in a seawater cooled condenser, a compressor has raised the pressure of the vapour to such an extent that at seawater temperature, it is supersaturated. Condensation takes place, and the latent heat released heats up the water passing through the condenser tubes; the heated seawater passing overboard into the sea, to be replaced continuously by fresh cool water. The resulting condensate will be somewhat warmer than the seawater coolant.
EVAPORATION: This is the process of converting a liquid into a vapour, and it requires latent heat to do this. If a liquid (say liquid propane) in a closed container at 10 °C. has a saturated vapour pressure of 5 atmospheres, and the vapour in the space above the liquid is allowed to escape, the pressure in the container will fall. As soon as this happens, the vapour in the space above the liquid will be undersaturated and evaporation will take place (or the liquid boil). Heat will be used up in the boiling process and the temperature of the liquid will fall. The “boil off” will largely replace the vapour which has been allowed to escape until such time as the pressure in the container corresponds to the saturated vapour pressure of the liquid at the new lower temperature. Continuous withdrawal of vapour means continuous evaporation, which in turn means continuous loss of heat (cooling).
FILLING OF CARGO TANKS: The correct maximum volume of liquid to load in a cargo tank is such a quantity that after allowance for the product to warm up and expand to a temperature the saturated vapour pressure of which would lift the safety valves, 2 per cent. of the space would remain.
A tank so filled is described as Full. A tank filled above this level is described as Overfull. A tank completely filled with liquid is described as one hundred per cent.
GAS/VAPOUR: Gas is a substance which has the property of indefinite expansion. In the context of this book, it is above its critical temperature and cannot be condensed into a liquid. If the temperature of a gas is reduced to below its critical temperature, it then becomes a vapour, and can be condensed into a liquid. Gases are frequently referred to as incondensibles.
Flammable or Explosive Mixture: Petroleum as a liquid does not burn. At ordinary temperatures, it gives off vapour, which when mixed within certain proportions with air, will burn. The lowest proportion of petroleum vapour in air mixture which will burn is termed lower explosive limit (LEL) and the strongest mixture that will burn is termed upper explosive limit (UEL). The flammable mixtures between the lower and upper explosive limits is called the explosive range. A mixture of vapour in air weaker than the LEL is described as too lean or over-lean whilst a mixture of vapour in air stronger than the UEL is described as too rich or over-rich. Mixtures outside the explosive range will not burn, the words explosive and flammable within this context being virtually synonymous.
Flash Point: This is the lowest temperature at which a flammable mixture of air and vapour will burn when exposed to a naked flame.
Ignition Temperature: This is the temperature at which a flammable mixture of vapour and air will ignite spontaneously (without being exposed to a naked flame). The operation of a diesel engine depends upon this effect.
Gas laws
Avogadro’s Hypothesis: Equal volumes of different gases at the same pressure and temperature contain the same number of molecules.
Boyle’s Law: The volume of a given mass of gas varies inversely with the pressure provided that the temperature remains constant:
Charles’s Law: The volume of a given mass of gas varies directly with the absolute temperature provided the pressure remains constant:
Clerk Maxwell’s Kinetic Theory: A gas may be imagined as a vast number of molecules moving in all directions at irregular velocities, colliding with one another and with the walls of the containing vessel. The path of a molecule is zig-zag in three dimensions and the mean free path is defined as the average length between collisions, the denser the gas, the shorter will be the mean free path.
On the assumption that the molecules are microscopic spheres, it can be shown that the pressure and absolute temperature of a gas are proportional to the mean kinetic energy of translation of the molecules bombarding the walls of the vessel containing the gas. Thus. at the same temperature the average kinetic energy of translation of the molecules of any gas is the same whatever its mass—a “large” molecule having low velocity and a “light” molecule having high velocity.
This theory correlates Avogadro’s Hypothesis. Boyle’s Law. Charles’s Law and Gay Lussac’s Law.
Dalton’s Law of Partial Pressures: The pressure of a mixture of gases is the sum of the pressures each would exert if it alone were to occupy the containing vessel.
Gay Lussac’s Law: The density of a gas at standard pressure and temperature is proportional to its molecular weight. This is a corrollary of Avogadro’s Hypothesis.
Joule’s Law: When a perfect gas expands without doing external work and without taking in or giving out heat and therefore without changing its stock of internal energy, its temperature does not change.
Heat
Latent Heat: This is the heat used up in changing the state of a substance without changing its temperature. In the case of changing the state of a substance from a solid into a liquid (melting), it is called the latent heat of fusion, and in the case of heat changing the state of a liquid into a gas or vapour (boiling), it is called the latent heat of vaporisation. It takes 80 calories to change 1 gramme of ice into water and about 539 calories to change 1 gramme of water into steam at standard atmospheric pressure. The value of latent heat of vaporisation varies with temperature and pressure (see critical temperature).
Sensible Heat: This is the heat used in raising the temperature of a substance without changing its state. 1 calorie is used to raise the temperature of 1 gramme of water 1 °C.
HEEL: This is the small quantity of liquid remaining after discharge which it is impossible to pump out, but which is used to assist in keeping the cargo tank cold during the ballast (unloaded) passage, and is usually carried over to the next loading. When it is known that the vessel will be changing grades or gas-freeing, every effort should be made to reduce this heel to the absolute minimum.
LIQUID CARRY OVER: This occurs when vapour moves swiftly over the surface of a liquid and droplets of liquid become entrained with the vapour and are carried over with it.
It is the entrained droplets of lubricating oil which are recovered in the lubricating oil separator trap of the compressors, and entrained liquid droplets which cause wet suction on a compressor.
MOLE: This is the quantity of gas the weight of which is equal to its molecular weight in pounds or grammes. Thus a mole of hydrogen would be 2, a mole of oxygen 32 etc. This is fairly closely related to Avogadro’s Hypothesis, a mole having the same volume for all products at the same pressure and temperature.
Pressure
Absolute Pressure: This is the pressure above a vacuum. Thus a pressure of 7 p.s.i. absolute, is really a suction pressure of 7.7 p.s.i. at atmospheric pressure (atmospheric pressure equals 14.7 p.s.i.).
Gauge Pressure: This is the pressure above one atmosphere and is the usual method of measuring pressures and vacuums. Absolute pressure is therefore equal to gauge pressure plus one atmosphere.
Atmospheric Pressure: This is the pressure exerted at sea level. This pressure varies from place to place and from time to time. The standard atmospheric pressure is 1012.5 millibars, corresponding to 29.90 inches or 760 millimetres of mercury.
SPAN GAS: This is a laboratory-measured mixture of gases used for the purpose of calibrating gas detectors. In gas tankers, the mixture is usually 30 per cent LEL of the product mixed with pure nitrogen.
STRATIFICATION: This is the layering effect of two gases or vapours with dissimilar densities, the lighter vapour floating above the heavier.
Temperature
Absolute temperature: As a result of studying Charles’s Law it seemed that the volume of a gas would reduce to nothing at about —273 °C. (or absolute zero). (Physicists have never been able to reach this temperature.) It therefore follows that absolute temperature equals temperature + 273 °C.
Adiabatic Changes in Temperature: When a gas (or vapour) is compressed, its temperature rises. When it expands, its temperature falls. This is the adiabatic process and compression ignition (diesel) engines rely upon this property for their operation.
Critical Temperature: This is the temperature above which it is not possible to liquify a gas. Saturated vapour pressure rises with an increase in temperature. At the same time, the density of a liquid falls with an increase in its temperature. Therefore, there must come a time when so many atmospheres of pressure are required to liquify the vapour that the density of the compressed vapour and the liquid are the same. When this state is achieved, there is virtually no difference between the liquid and vapour phases and they freely change into each other. The value of latent heat is reduced to zero and with any increase in temperature, no amount of increasing the pressure will bring about liquifaction, and the vapour is then described as a gas. Associated with the critical temperature is the critical pressure.
VAPORISATION: This is the action of converting a liquid into a vapour.
Batch Vaporisation: This is the method of evaporation whereby vapour is withdrawn from the top of a tank, causing the liquid in the tank to boil, with a consequent drop in temperature. Also, with a mixture of products such as butane and propane, the more volatile element tends to evaporate first, so that the proportions comprising the mixture will change and after a time one is left with almost pure butane. This process of altering a mixture in a tank due to the volatile constituent evaporating first is called “weathering”. However, batch vaporisation is the simplest method and because, in LPG tankers, the vapour which has been withdrawn is condensed into a liquid and returned to the tank, there is no tendency to alter the constituents of the mixture, so this is used as a method of refrigeration.
Flash Vaporisation: This is the method whereby liquid is withdrawn from the bottom of the tank and evaporated in a vaporising unit. In this method, the constituents of a mixture remain fairly constant, as does the temperature of the product in the tank.
VAPOUR: This is the term used for a “gas” below its critical temperature and therefore capable of being liquified.
Saturated Vapour Pressure (SVP) All liquids tend to evaporate under normal conditions, but if kept in a closed container, evaporation will only take place until the atmosphere in the container becomes saturated. In the case of water, the following experiment can be carried out. Into the top of a barometer some water is introduced. Due to the evaporation of the water that has been introduced, the level of the mercury will fall. If sufficient water is introduced, the level will virtually stop falling because the space above the mercury will be saturated with water vapour, and a little water will show on top of the mercury. The fall in the mercury level converted into pressure would indicate the absolute SVP at that temperature. By raising the temperature, more water will evaporate and the level of the mercury fall further. The new level, converted into pressure, will indicate the new SVP at the new temperature. At 100 °C., the level of the barometer will register zero. The absolute vapour pressure of water at 100 °C. is therefore one atmosphere (1.0125 bar). It therefore follows that under atmospheric conditions, a liquid will, apart from minor evaporation, keep its state until with the addition of heat, its absolute SVP reaches one atmosphere. From then on, all the extra heat will be used to assist evaporation and the temperature will not rise. In other words, the liquid boils. If the boiling action takes place in a closed container, e.g. a boiler, as the temperature rises, so the pressure increases. That is, the boiling temperature of the water rises as the pressure increases. The pressure in the boiler is an indication of the water temperature and vice versa.
If a thermometer and pressure gauge were fitted to a container holding, say, propane, the temperature and pressure would be directly related to each other, the pressure rising as the temperature rose and vice versa.
A sudden release of pressure would result in continuous evaporation, this using up latent heat so cooling the liquid until the temperature of the liquid reached that appropriate to the SVP of the product at the new pressure. This means that if warm propane escaped onto the deck, it would immediately evaporate and refrigerate itself down to approximately —43 °C.
Supersaturated Vapour: If the vapour pressure in a container is rapidly increased, condensation will take place, but until the process of condensation has been completed, the vapour will be supersaturated.
Undersaturated Vapour: This is supersaturation in reverse.
Superheated Vapour: In the absence of liquid to continue the evaporating process and so keep the vapour saturated, the vapour temperature can be raised to well above the temperature corresponding to that at which the vapour would be saturated at the pressure concerned. Any superheated vapour would have no tendency to condense. This property is used particularly with steam. The saturated steam coming from the boilers is heated further in the superheater to prevent condensation taking place in the engine.
VAPOUR RETURN LINE: This is a balancing pipeline between the ship when loading (or discharging) and the shore tank, so that the vapour trapped in the space above the incoming liquid, and therefore being compressed, is returned to the shore tank from which the product is being discharged.
ZERO GAS: This is pure nitrogen used to calibrate the zero reading of gas detectors.

Формулы сломались, исправьте пожалуйста =)
Добрый день! Исправил, благодарю за сигнал.