The chemical and physical properties of liquefied gas are unique. These properties include being flammable, odorless, colorless, and heavier than air. They can evaporate quickly in air, forming clouds of flammable gas. Liquefied gases are stored and transported under controlled conditions to prevent accidents and ensure safety.
Physical properties of cargo refer to characteristics such as density, viscosity, melting point, boiling point, and Flash Point – Definition and Pronunciationflash point. These properties are important when handling and transporting different types of cargo, including liquids, gases, and solids. Understanding the physical properties of cargo helps ensure safe and efficient cargo operations and compliance with regulations.
Liquefied Gas
A liquid which has a saturated vapor pressure exceeding 2,8 bar absolute at 37,8 ⁰C and certain other substances specified in the Gas Codes.
Chemical Structure of Gases
Chemical compounds with the same chemical structure are often known by different names. An alternative name given to the same compound is called a synonym. Since a molecule is the smallest part of compound which exhibits all the chemical properties of that specific material, this formula is often referred to as the molecular formula.
Hydrocarbons are substances whose molecules contain only hydrogen and carbon atoms. The molecules can be in various arrangements and the products may be gases, liquids or solids at ambient temperatures and pressures, depending upon the number of the carbon atoms in the molecular structure:
- Generally, those hydrocarbons with up to four carbon atoms are gaseous at ambient conditions and comprise the hydrocarbon liquefied gases.
- Hydrocarbons with five up to about twenty carbon atoms are liquid at ambient conditions.
- Those with more carbon atoms are solid.
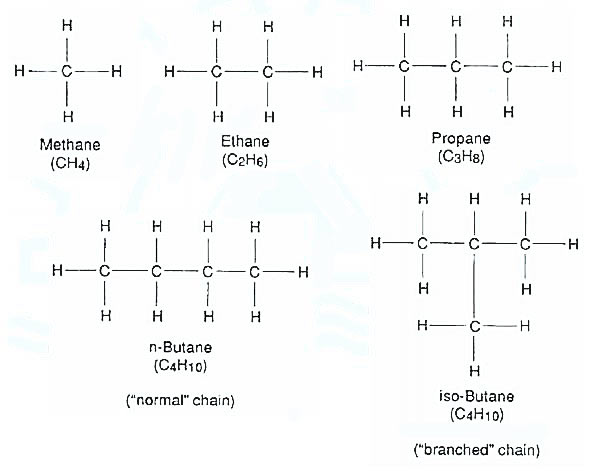
The carbon atom has four bonds which can unite with other carbon atoms or with atoms of other elements. A hydrogen atom, however, has only one bond and can unite with only one other atom. Where the relative numbers of carbon and hydrogen atoms in a hydrocarbon molecule permit the carbon atoms to use their bonds singly to other carbon atoms, the molecule is said to be saturated. Examination of these examples shows that, for saturated hydrocarbons, the proportion of carbon and hydrogen atoms in the molecule is in accordance with the formula CnH2n + 2. Thus, methane (CH4), ethane (C2H6), and propane (C3H8) are all saturated hydrocarbons.
Where there is less than the full complement of hydrogen atoms, as given by the above formula, two or more carbon atoms become inter-linked by double or triple bonds. For this reason they are called unsaturated. These links between carbon atoms are weaker than single bonds with the result that such compounds are chemically more reactive than the single-bonded compounds.
The molecular structure of two such unsaturated hydrocarbons, propylene (C3H6), and butadiene (C4H6). Ethylene (C2H4) is a further example of an unsaturated hydrocarbon.
The third Safety Liquefied Gas of Marine Transportationgroup of liquefied gases consists of the chemical gases. These are characterized by additional atoms other than carbon and hydrogen. Most compounds in this grouping are chemically reactive.
Saturated hydrocarbons
The saturated hydrocarbons are:
- Methane.
- Ethane.
- Propane.
- Butane.
These are all colorless liquids and odorless. They are all Fire Suppression Systems for Liquefied Gas Carriersflammable gases and will burn in air or oxygen to produce carbon dioxide and water vapour. They do not present chemical compatibility problems when in contact with the construction materials commonly encountered in gas handling. In the presence of moisture, however, the saturated hydrocarbons may form hydrates.
Unsaturated hydrocarbons
The unsaturated hydrocarbons are:
- Ethylene.
- Propylene.
- Butylenes.
- Butadiene.
- Isoprene.
These are colorless liquids with a faint, sweetish odor. Like the saturated hydrocarbons they are all flammable in air or oxygen, producing carbon dioxide and water vapour. They are more reactive, from a chemical viewpoint, than the saturated hydrocarbons and may react dangerously with chlorine. Ethylene, propylene and butylene do not present chemical compatibility problems with materials of construction, whereas butadiene and isoprene, each having two pairs of double bonds, are by far the most reactive within this family. They may react with air to form unstable peroxides which tend to induce polymerization. Butadiene is incompatible in the chemical sense with copper, silver, mercury, magnesium, aluminium and monel. During production, butadiene streams often contain traces of acetylene which can react with brass and copper to form explosive acetylides.
Water is soluble in butadiene, particularly at high temperatures. As can be seen, on cooling water-saturated butadiene the solubility of the water decreases and water will separate out as droplets which settle as a layer in the bottom of the tank. For instance, on cooling water-saturated butadiene from +15 °C to +5 °C approximately 100 parts per million of free water separates out. On this basis, for a 1 000 m3 tank, 0,1 m3 of free water would require to be drained from the bottom of the tank. On further cooling to below zero this layer of water would increase in depth and freeze.
Cargo physical properties
- Temperature.
- Pressure.
- Volume Expansion.
- Viscosity.
- Specific Gravity.
- Vapor Density.
- Solubility in water.
- Electrostatic Generation.
Vapour Pressure/Temperature Relationship
Vapour pressure is directly proportional to temperature. Vapour pressure increases with increasing temperature and decrease with decreasing temperature.
Vapour Pressure – of pure compound depends only upon its temperature and a mixture depends both upon its temperature, and the volume of the gas space in which vaporization occurs.
Influence of pressure on boiling temperature
Boiling point takes place in a liquid when the vapour pressure is equal to the pressure in the liquid. Varying the pressure above the liquid (atmosphere Pressure) it is possible to boil the liquid at different temperature. When decreasing the pressure above the liquid lowers the boiling point and increasing the pressure raises the boiling point.
Saturated vapour pressure – the pressure at which a vapour is in equilibrium with its liquid at a specified temperature.
Vapour in the space above the liquid is not static since liquid molecules near the surface are constantly leaving to enter the vapour phase and vapour molecules are returning to liquid phase. The space is said to be «unsaturated» with vapour at a particular temperature if the space can accept more vapour from the. In that condition the space cannot accept any further vapour from the liquid, although a continuous exchange of molecules between vapour and liquid takes place.
True Vapour Pressure – the vapour pressure of a liquid is the absolute pressure exerted by the gas produced by evaporation of a liquid when gas and liquid are in equilibrium at the prevailing temperature and gas liquid/gas ratio is effectively zero. The true vapour pressure (TVP) of petroleum is difficult to measure but the correlation exists between (TVP) & Reid Vapour Pressure.
Reid Vapour Pressure (RVP) – the vapour pressure of a liquid determined by laboratory testing in a standard manner in the Reid apparatus at a standard temperature of 100 °C (37,7 °C) expressed in pounds per square inch absolute and commonly written as «RVP…. lb».
Saturated Hydrocarbons – Where relative numbers of hydrogen & carbons in a hydrocarbon molecule permit the carbon atom to use their bond singly to other carbon atom. Examples are:
- Methane.
- Ethane.
- Propane.
- Butane.
Chemical properties of saturated hydrocarbon
They are all flammable gases will burn in air and or oxygen to produce carbon dioxide and vapour they chemically non-reactive and do not present chemical compatibility problems with material commonly used in handling. In presence of moisture they may form hydrates.
Unsaturated Hydrocarbon – Where there is less that the full complement of hydrogen atoms, two or more carbon atoms become interlinked by double or perhaps triple bond. Examples are ethylene, propylene and butylene, butadiene and isoprene are colourless liquids with faint, sweetish characteristic odours.
Chemical properties of unsaturated hydrocarbon
They are also flammable in air, producing carbon dioxide and water vapour. They are chemically more reactive and may react dangerously with chlorine, ethylene, propylene and butane. Do not present chemical compatibility problem with materials of constructions while butane and isoprene are the most chemically reactive within this family.
Read also: Gas laws, thermodynamic principles and reliquefaction
Diffusion – the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane or microporous barrier. Substance ability to mix and become uniform.
Volatility – Tendency of a solid or liquid to pass into vapour state at a given temperature. Specially the vapour pressure of a component divided by its mole fraction in liquid or solid. Is characterized by the vapour pressure. When petroleum is transferred in tank or container that is gas free it begins it vaporize and liberate gas into the above and also a tendency of liberation or releasing gases to be dissolved into the liquid where equilibrium is reach and gases are evenly distributed in the space.
Volatile – liquids that evaporate readily are known as volatile liquids. Any petroleum with a flashpoint below 60 °C (140 °F) is closed as volatile. Refinery waste-spent caustic soda for example may contain volatile petroleum.
Non Volatile – Some other petroleum evaporates less rapidly, those with a flashpoint of 60 °C (140 °F) or over and as non – volatile. Gas oil and diesel oil are two examples.
Flash Point – lowest temperature at which a flammable substance will give off vapour that will ignite when a flame or spark is introduced in the presence of sufficient oxygen.
Flammability – the ability of Hydrocarbon Process Safety – Fire Hazards, Risks and Controlshydrocarbon gases to react with oxygen in the air to produced carbon dioxide and water. This reactions gives enough heat to form a visible flame which travel through the mixture of hydrocarbon gas and air.
Flammability – Limits and Range
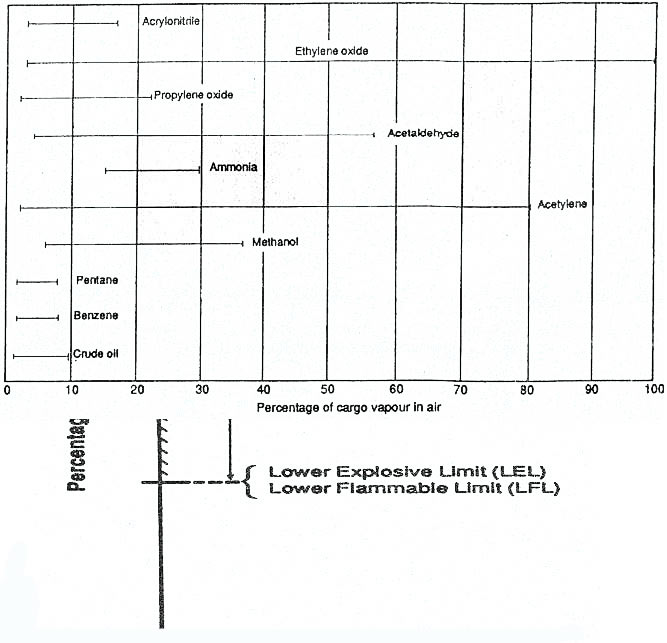
Flammability Limit – the limit in which hydrocarbon gas and air cannot ignite and burn unless its composition lies with in a range of gas in air concentration in which there is sufficient hydrocarbon gas to support and propagate combustion.
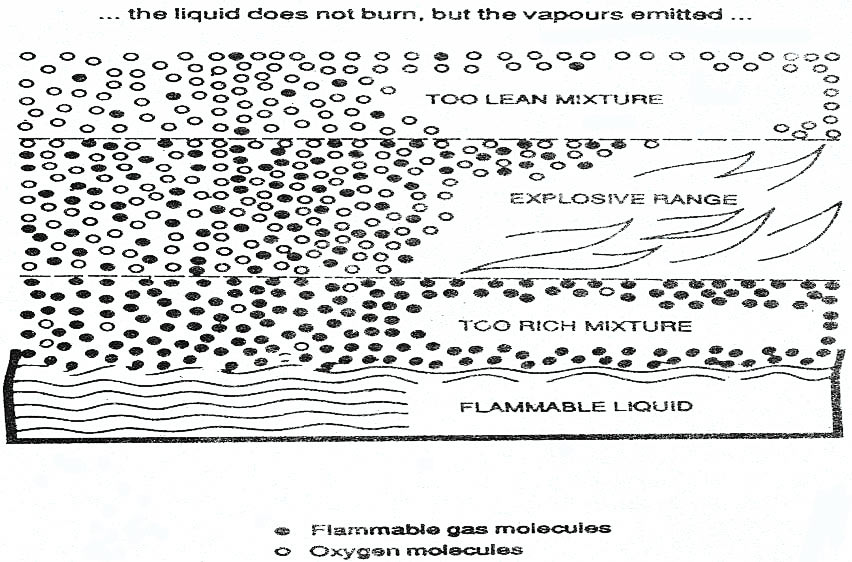
Upper Flammable Limit (UFL) – The upper limit range of flammability.
Lower Flammable Limit (LFL) – The lower limit range of flammability.

