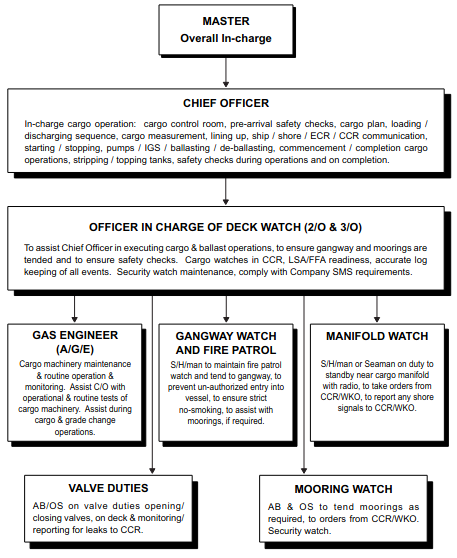
Responsibilities of personnel on the LPG tankers are crucial for ensuring the safe and efficient operation. These responsibilities encompass a range of roles, including the Master, Chief Officer, Second and Third Officers, Chief Engineer, Gas Engineer, and Ratings. Each member plays a vital part in maintaining safety protocols and managing the handling of liquefied petroleum gas.
Understanding the physical and chemical properties of LPG, as well as principles of refrigeration and thermodynamics, is essential for effective cargo management. Overall, the collective efforts of LPG personnel are fundamental to the safe transportation and storage of this important energy resource.
Responsibilities
In the operation of any vessel it is essential that good co-operation exists between all personnel on board. In addition to the responsibilities defined in the SMS manual, job descriptions contained herein concerning cargo operations are to be expounded as follows:
Master
The Master is responsible for:
- Ensuring that the voyage orders are fully understood and complied with.
- Advising the Chief Engineer and Chief Officer of the relevant details of the intended voyage.
- Providing the Company and Charterers with accurate information regarding the cargo operations.
- Advising the Company when instructions cannot be complied with or he is unsure of the intended voyage instructions.
- Overall supervision of the operation of cargo system.
- Checking and verification of cargo plans prepared by the Chief Officer.
- Overall supervision of all cargo and ballast operations onboard.
- Completion, checking and signing as applicable of cargo documents.
- Maintaining the expertise of all ships staff in the gas carrier field.
Chief Officer
Under the supervision of the Master the Chief Officer is responsible for:
- Preparation of the cargo plan in compliance with the voyage orders.
- Checking of the cargo system to ensure that the intended plan will be followed.
- Preparation of the ship’s cargo and ballast operations onboard and personal supervision of these operations.
- Operation and control of all cargo equipment.
- Producing his own written standing orders concerning the cargo operations which are to be well understood and signed by each deck officer.
- For monitoring of the vessel’s stress and stability throughout the cargo and ballast operations and during the voyage to ensure that they remain within the required limits.
- Maintaining Cargo Records as required by the Company, Charterers and International Regulations.
- Calculation of the cargo quantity on board and preparation of cargo documentation as required.
- Checking of all compartments on a daily basis and recording the sounding in the deck log book.
- For monitoring of toxic gases vapor concentration in ship’s compartments as required.
- Maintaining the records required in conjunction with the Chief Engineer as required.
- Applying the ballast water management practices required and maintaining records.
Second Officer and third Officer (Including additional Officer)
The Second and Third Officers are responsible to the Chief Officer for:
- Assisting in all cargo, ballast systems preparation.
- Monitoring of cargo and ballast operations as instructed by the chief officer.
- Ensuring that a proper deck and security watch is maintained.
- Ensuring that the vessel remains securely moored at all times.
- Complying with Company’s SMS requirements, checks & record keeping.
Chief Engineer
The Chief Engineer is overall responsible for the maintenance and repair of all the ship’s Cargo and Ballast related equipment. He is to assist the Chief Officer in the operation of the ship’s cargo equipment. He is to keep the Chief Officer advised of any bunker, lubricating oils or sludge transfers which may affect the ship’s trim, stress and/or stability.
Gas Engineer
The Gas Engineer’s Salariesgas engineer must maintain the equipment and machinery under his charge in good order. He must also carry out control of temperature and pressure of cargo tanks and control of cargo in accordance with the chief officer’s orders. He must investigate, study and be thoroughly familiar with the LPG plant and specially with the construction, operating principle, piping system, running conditions and other factors of the machinery and equipment under his charge, also after joining the ship he must promptly familiarize himself with the normal operating methods and regular duties pertaining to the machinery and equipment coming under his charge. Gas engineer is responsible for:
- LPG control and maintenance;
- operation and control of LPG equipment in general;
- various CCR machinery and equipment;
- IGG and related equipment;
- gas compressors;
- heat exchangers for cargo;
- LPG instrumentation;
- automatic control devices of equipment in his charge;
- gas detector system;
- control of related parts and spares;
- control of fixtures and ship stores;
- handling documents and office work.
Ratings
The deck ratings are responsible to the deck officers for maintaining a safe deck watch and ensuring that the vessel remains securely moored. They are to assist in cargo operations as required by the responsible officer.
Note: Nothing contained in this section shall be construed as relieving the Master or Officers of their responsibility as defined by law, or relieving them from the exercise of sound judgement and authority at all times for the purpose of safety & security.
Introduction to Gas Tankers
Physical Properties
The physical properties of a liquefied gas depend on its molecular structure. Some compounds have the same molecular formula but a different arrangement within the structure. These different compounds of the same basic substance are called ISOMERS for example N-BUTANE & ISO-BUTANE.
The single most important physical property of a liquefied gas is its saturated vapor pressure/temperature relationship. This property governs the design of the containment system suitable for each cargo.
Liquefied Gases. A gas is a collection of molecules moving freely in space. In a closed container the speed at which the molecules move depends upon the energy available or the temperature of the gas. If the gas is cooled down (chilled), the speed at which the molecules move will decrease and, eventually, they will combine to form a liquid.
A liquefied gas is the liquid form of a substance which, at ambient temperature and at atmospheric pressure, would be a gas.
The IMO for the purposes of its Gas Carrier Codes relates saturated vapour pressure to temperature and has adopted the following definition for the gases carried by sea:
Liquids with a vapour pressure exceeding 2,8 bar absolute at a temperature of 37,8 °C.
In other word a Chemical Composition and Physical Properties of Liquefied Gasesliquefied gas is to give the temperature at which the saturated vapour pressure is equal to atmospheric pressure i. e. the liquid’s atmospheric boiling point.
| Table 1. Physical Properties of some liquefied gases | ||
|---|---|---|
| Liquefied Gas | Vapour Pressure at 37,8 °C (bars absolute) | Boiling point at atmospheric pressure (°C) |
| Methane | Gas | -161,5 |
| Propane | 12,9 | -42,3 |
| n-Butane | 3,6 | -0,5 |
| Ammonia | 14,7 | -33,4 |
| Vinyl Chloride | 5,7 | -13,80 |
| Butadiene | 4 | 5,00 |
| Ethylene Oxide | 2,7 | 10,70 |
LPG Production. Liquefied Petroleum Gas (LPG) is largely given name for propane, butane or mixture of the two. These products are extracted from natural gas or crude oil streams during the initial processing, and it is obtained during the refining of crude oil.
The vapour of both propane & butane are heavier than air. Propane vapour forms 270 times that of liquid & butane 230 times or in other words a single drop of propane liquid will occupy 270 times space when in vapour form & butane will occupy 230 times.
Chemical & Physical properties of Liquefied gases. Hydrocarbons are substances whose molecules only contain only hydrogen & carbon atoms. The molecule can be in various arrangements and products may be gases, liquid or solids at ambient temperature and pressures, depending upon the number of the carbon atoms in the molecular structure. Generally, those hydrocarbons with up to four carbon atoms are gaseous state at ambient conditions and comprise the hydrocarbon liquefied gases. Hydrocarbons with five up to twenty carbon atoms are liquid at ambient conditions and those more carbon atoms are solid.
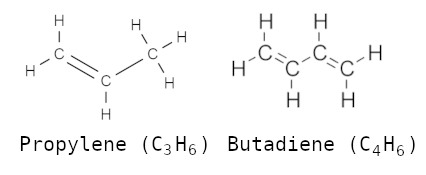
As per the bonding of carbon atoms they form saturated hydrocarbons (CnH2n+2) like, methane (CH4), propane (C3H8) & butane (C4H10), and unsaturated hydrocarbons like ethylene (C2H4), propylene (C3H6) & butadiene (C4H6).

Molecular weight of propane is about 44 and that of butane is about 58, which means that they are heavier than air. Molecular weight of air is calculated to be about 29, therefore propane is about 1.5 times and butane is 2 times as heavy as air. These gases if leaked into atmosphere will accumulate in low places. Therefore, extensive detection of gas in low places when leakage is found is necessary.
Risks and Compliance for Environment for Liquefied Petroleum Gas Operations within Inland WaterwaysGas leaking from the upper deck may flow into the engine room compartment by way of accommodation openings when there is no wind. Therefore closing of entrances and portholes on upper deck during cargo operations is required.
Propane and butane in liquid phase are both about 0,6 in specific gravity, which is half as heavy as water. Therefore, if water is contained in the cargo, it will settle down to the tank bottom and can cause danger to cargo pump.
Flammability. A correct proportion of mixture of air & hydrocarbon is required ignite a hydrocarbon gas. When the concentration of a hydrocarbon gas in air below which there is insufficient hydrocarbon to support combustion is called as Lower Flammability Limit (LFL) and when the concentration of a hydrocarbon gas in air above which there is insufficient air to support combustion, is called as Upper Flammability Limit (UFL).
Flammable Range (or Explosive Limit). Explosive limit is the limit of concentration of gas in air by which explosion occurs if ignited. There are upper Explosive limit (upper flammable limit) and Lower Explosive limit (Lower Flammable Limit), that means no explosion n occurs if there is too rich gas or too lean gas in air.
Their LEL being about 2 % means that there is only 2 % of gas in air, explosion can occur. As their specific gravity is greater than air, explosive mixture will be accumulate at lower places on deck.
| Table 2. Explosive limit with Air (%) | ||
|---|---|---|
| Lower Limit | Upper Limit | |
| Propane | 2,37 | 9,50 |
| n-Butane | 1,86 | 8,41 |
Explosive limit/or range changes as the contents of the gas mixture changes.
Other Properties. Propane, butane & LPG are colourless and odourless gases. These are transported at both their liquid & gas phases, however, if the gas is seen in air, different coefficient of refraction makes it to like haze, even though these are colourless gases.
Therefore if gas is emitting from vent riser it can be clearly observed from the wheelhouse with the help of binoculars, enabling the ship personnel to detect gas emission. Pure propane & butane are odourless, however commercial products are stenched enabling detection in case of leak.
Propane & butane are not very toxic, but they are harmful if inhaled. These gases normally do not react with water but forms a greasy type solid called hydrate.
Chemical Properties
Chemical compounds with the same chemical structure are often known by different names. The alternative names given to the same compound is called a synonym. The table below gives a list of the synonyms of the liquefied gases against each common name and its simple formula. The more complex compounds tend to have a larger number of synonyms than the simple compounds.
| Table 3. Synonyms of liquefied gases | ||
|---|---|---|
| Common Name | Simple Formula | Synonyms |
| Methane | CH4 | Fire damp; marsh gas; natural gas; LNG |
| Ethane | C2H5 | Bimethyl; dimethyl; Methyl Methane |
| Propane | C3H8 | |
| n-Butane | C4H10 | Normal Butane |
| i-Butane | C4H10 | Iso-Butane; 2-Methlypropane |
| Ethylene | C2H4 | Ethene |
| Propylene | C3H6 | Propene |
| a-Butylene | C4H8 | But-1-ene; Ethyl ethylene |
| b-Butylene | C4H8 | But-2-ene; dimethyl ethylene; Pseudo butylenes |
| y-Butylene | C4H8 | Isobutene; 2-Methylprop-2-ene |
| Butadiene | C4H6 | b.d.; bivinyl; 1,3 butadiene; butadiene 1-3; bivinyl; biethylene; erythrene; vinyl ethylene. |
| Isoprene | C5H8 | 3-methyl – 1,3 butadiene; 2-methyl – 1,3 butadiene; 2-methylbutadiene – 1,3 |
| Vinyl Chloride Monomer | CH2CHC1 | Chloroethene, Chloroethylene; VCM |
| Ethylene oxide | C2H4O | Dimethylene oxide; EO; 1,2 epoxyethane; Oxirane |
| Propylene oxide | C3H6O | 1,2 epoxy propane; methyl oxirane; Propene oxide |
| Ammonia | NH3 | Anhydrous ammonia; ammonia gas; liquefied ammonia; liquid ammonia |
| Note: Commercial propane contains some butane; similarly commercial butane contains some propane. Both may contain impurities such as ethane and pentane, depending on their permitted commercial specification. | ||
Saturated hydrocarbons. The saturated hydrocarbons methane, ethane, propane and butane are all colorless and odorless liquids under normal conditions of carriage. They are all flammable gases and will burn in air and/or oxygen to produce carbon dioxide and water vapor. As they are chemically non-reactive they do not present chemical compatibility problems with materials commonly used in handling. In the presence of moisture however, the saturated hydrocarbons may form hydrates. Sulphur compounds such as mercaptans are often added as odourizer prior to sale to aid the detection of these vapors. This process is referred to as stenching. Mercaptans are considered to be hazardous in some forms and must be handled with care when on board the vessel.
Unsaturated hydrocarbons. The unsaturated hydrocarbons ethylene, propylene, butylene, butadiene and isoprene are colorless liquids with a faint, sweetish characteristic odor. They are, like the saturated hydrocarbons, all flammable in air and/or oxygen, producing carbon dioxide and water vapor. They are chemically more reactive than the saturated hydrocarbons and may react dangerously with chlorine. Ethylene, propylene and butylene do not present chemical compatibility problems with materials of constructions, whereas butadiene and isoprene, each having two pairs of double bonds, are by far the most chemically reactive within this family group. They may react with air to form peroxides which are unstable and tend to induce polymerization. Butadiene is incompatible in the chemical sense with:
- copper;
- silver;
- mercury;
- magnesium;
- aluminium and monel.
Butadiene streams often contain traces of acetylene which can react to form explosive acetylides with brass and copper.
Water is soluble in butadiene, particularly at elevated temperatures. The figures quoted are for the purpose of illustration only. On cooling water-saturated butadiene the solubility of the water decreases and water will separate out as droplets which will settle as a layer on the bottom of the tank, for instance, on cooling water-saturated butadiene from +15 °C to + 5 °C approximately 100 ppm of free water would separate out. On this basis, for a 1 000 m tank, 100 c of free water would require to be drained from the bottom of the tank. On further cooling to below 0 °C this layer of water would increase in depth and freeze.
Chemical gases. The chemical gases commonly transported in liquefied gas carriers are ammonia, vinyl chloride monomer, ethylene oxide, propylene oxide and chlorine. Since these gases do not belong to one particular family their chemical properties vary.
Liquid ammonia is a colorless alkaline liquid with a pungent odor. The vapors of ammonia are flammable and burn with a yellow flame forming water vapor and nitrogen, however, the vapor in air requires a high concentration (16-25 percent) to be flammable, has a high ignition energy requirement (600 times that for propane) and burns with low combustion energy. For these reasons the IMO Codes, while requiring full attention to the avoidance of ignition source, do not require flammable gas detection in the hold or inter-barrier spaces of carrying ships. Nevertheless, ammonia must always be regarded as a flammable cargo.
Ammonia is also toxic and highly reactive. It can form explosive compounds with:
- mercury;
- chlorine;
- iodine;
- bromine;
- bromine;
- calcium;
- silver oxide;
- and silver hypochlorite.
Ammonia vapor is extremely soluble in water and will be absorbed rapidly and exothermically to produce a strongly alkaline solution of ammonium hydroxide. One volume of water will absorb approximately 200 volumes of ammonia vapor. For this reason it is extremely undesirable to introduce water into a tank containing ammonia vapor as this can result in vacuum condition rapidly developing within the tank.
Since ammonia is alkaline, ammonia vapor/air mixtures may cause stress corrosion. Because of its highly reactive nature copper alloys, aluminium alloys, galvanized surfaces, phenolic resins, polyvinyl chloride, polyesters and viton rubbers are unsuitable for ammonia service. Mild steel, stainless steel, neoprene rubber and polythene are, however, suitable.
Vinyl chloride monomer (VCM) is a colorless liquid with a characteristic sweet odor. It is highly reactive, though not with water, and may polymerize in the presence of oxygen, heat and light. Its vapors are both toxic and flammable. Aluminium alloys, copper, silver, mercury and magnesium are unsuitable for vinyl chloride service. Steels are, however, chemically compatible.
Toxide and propylene oxide are colorless liquids with an ether-like odor. They are flammable, toxic and highly reactive. Both polymerize, ethylene oxide more readily than propylene oxide, particularly in the presence of air or impurities. Both gases may react dangerously with ammonia.
Cast iron, mercury, aluminium alloys, copper and alloys of copper, silver and its alloys, magnesium and some stainless steels are unsuitable for the handling of ethylene oxide. Mild steel and certain other stainless steels are suitable as materials of construction for both ethylene and propylene oxides.
Nitrogen Padding is Required. Chlorine is a yellow liquid which evolves a green vapor. It has a pungent and irritating odor. It is highly toxic but is non-flammable though it should be noted that chlorine can support combustion of other flammable materials in much the same way as oxygen. It is soluble in water forming a highly corrosive acid solution and can form dangerous reactions with all the other liquefied gases. In the moist condition, because of its corrosivity, it is difficult to contain. Dry chlorine is compatible with mild steel, stainless steel, monel and copper. Chlorine is very soluble in caustic soda solution which can be used to absorb chlorine vapor.
Principles of Refrigeration
Cold liquid refrigerant (liquid gas cargo) is evaporated in an evaporator coil (cargo tank) which being cooler than its surroundings draws heat to provide the latent heat of vaporization. The cool vapor is drawn off by a compressor which raises both the pressure and the temperature of the vapor and passes it to the condenser. The pressure of the vapor having been increased the vapor now has a temperature of condensation greater than the temperature of the condenser cooling fluid (sea water).
The vapor is condensed to a high pressure liquid and the sensible heat of de-superheating the vapor together with the latent heat of condensation is removed via the condenser coolant which is warmed up in the process.
The high pressure liquid then passes through an expansion valve to the low pressure side of the cycle, in doing so flash evaporates to a mixture of cold liquid and vapor. The mixture passes to the evaporator (cargo tank) to continue the cycle.
Critical Temperatures & Pressures
The critical pressure of a gas is the pressure required to compress a gas to its liquid state at its critical temperature.
Read also: Cargo equipment for gas carriers carrying LNG/LPG
Critical temperatures and pressures are listed in the table of Physical Properties. For the carriage or storage of ethane or ethylene as a liquid some additional refrigeration is required often in the form of a cascade system.
Basic Thermodynamic Theory
Cargo and Deck Officers must be familiar with Basic Thermodynamic Theory. Section 13.4 and A3.5 of the Tanker Safety Guide – Liquefied Gas, dealing with the most important points and these sections are to be read in conjunction with this manual.
Cargo Information
The IMO Codes require that the following information is available to every ship and for each cargo:
- A full description of the physical and chemical properties necessary for the safe containment of the cargo;
- Action to be taken in the event of spills or leaks;
- Counter-measures against accidental personal contact;
- Fire-fighting procedures and fire-extinguishing agents;
- Procedures for cargo transfer, gas-freeing, ballasting, tank cleaning and changing cargoes;
- Special equipment needed for the safe handling of the particular cargo;
- Minimum inner hull steel temperatures;
- Emergency procedures;
- Compatibility;
- Details of the maximum filling limits allowed for each cargo that may be carried at each loading.
Temperature, the maximum reference temperature, and the set pressure of each relief valve. This above information is usually obtained from the cargo material safety data sheets (MSDS). Refer to the appendix for the «Cargo data sheets» for Propane and Butane.
The Master is to request the correct technical name of the cargo to be loaded as soon as possible before loading. If the cargo is not adequately covered by a data sheet, he is to obtain sufficient additional information necessary for its safe carriage.
The Master and all those concerned are to use the data sheet and any other relevant information to acquaint themselves with the characteristics of each cargo to be loaded. If the cargo to be loaded is a mixture (e. g. LPG), information on the composition of the mixture is to be sought; the temperature and pressure readings in the shore tank can be used to verify this information.
If the information necessary for safe carriage is not available, loading is to be refused; loading must also be refused if a cargo has to be inhibited but no certificate is available. Special note is to be made of any contaminants that may be present in the cargo, e. g. water.

