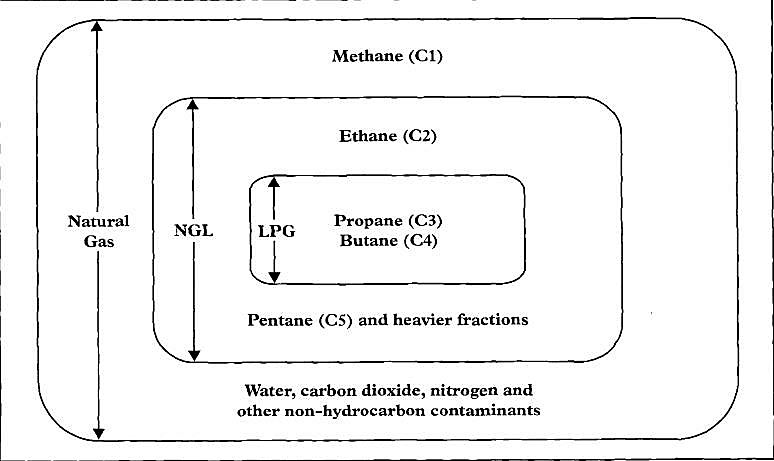
Natural gas chemical properties affect the characteristics of its transportation and use. Natural gas is primarily composed of methane, but may also contain small amounts of other gases such as ethane, propane, butane and pentane. It is a flammable gas that is colorless and odorless.
To detect leaks, an odorant called mercaptan is often added, which gives natural gas a characteristic «rotten egg» smell. Delivery of liquefied gas to the consumer is a very complex and labor-intensive process. After liquefying the gas at plants, LNG enters storage facilities. Further transportation is carried out using special vessels – gas carriers equipped with cryotankers.
Liquefied petroleum gas (LPG) – origins and characteristics
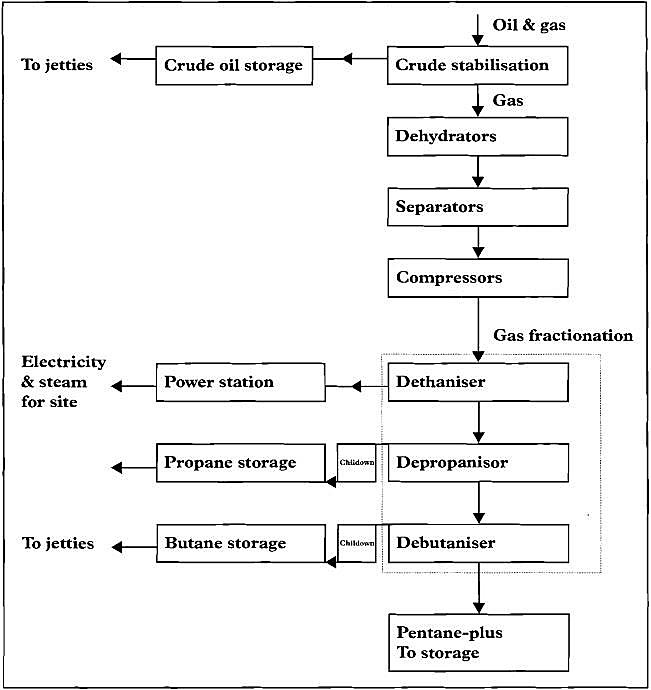
Liquid Petroleum Gases, known as LPG, is the generic name used in the oil industry for butane (C4H10), propane (C3H8) or a mixture of the two. It describes odourless, non-toxic hydrocarbons which exist as a gas at normal atmospheric pressure and an ambient temperature of 15,6 °C. These products are currently obtained by three methods:
- The treatment of crude oil to remove volatile components including methane, ethane, propane and butane. This process «stabilises» the crude oil prior to movement by pipeline or tanker.
- The refining of crude oil by distillation or cracking allows further amounts of butane and propane to be released from «stabilised» crude oil.
- The treatment of wet natural gas from natural gas or crude oil streams coming from underground reservoirs located within petroleum producing countries.
LPG manufactured from refinery cracked gases represents approximately 38 % of global supplies, the remaining being extracted from the processing of associated or non-associated natural gas.
LPG is one of the cleanest fuels available for domestic, commercial or industrial markets, with a very low sulphur content. This is the main reason for its original development. However, several secondary usages have since generated large markets; i-butane and n-butane are important octane enhancers for motor gasoline and key petrochemical feedstocks. Propane is used as fuel for power generation, for industrial purposes and as petrochemical feedstock.
Liquefied Petroleum Gases are readily condensable to liquid at moderate pressures and temperatures. By liquefying the gas, its volume is reduced by a factor of 250. They are lighter than water when in liquid form with a density of 0,583 kg/m3 for propane and approximately 0,600 kg/m3 for butanes. They can be shipped in pressurised condition at ambient temperature or fully refrigerated at atmospheric pressure at temperatures of -0,5 °C for n-butane, -11,7 °C for i-butane and -42,3 °C for propane. A mixture of 2,1 to 9,4 % of propane and 1,8 to 8,5 % of butanes gas in air can catch fire when in contact with a spark or a naked flame. When LPG is exposed to ambient temperature and atmospheric pressure, it vaporises quickly, and the gas is between 1,5 and 2 times heavier than air. LPG is colourless at ambient temperature and odourless.
| Liquefied gas | Vapour pressure at 37,8 °C (bars absolute) | Boiling point at atmospheric pressure (°C) |
|---|---|---|
| Methane | Gas* | -161,5 |
| Propane | 12,9 | -42,3 |
| n-butane | 3,6 | -0,5 |
| Ammonia | 14,7 | -33,4 |
| Vinyl chloride | 5,7 | -13,8 |
| Butadiene | 4 | -5 |
| Ethylene oxide | 2,7 | 10,7 |
| * The critical temperature of methane is -82,5 °C while the critical pressure is 44,7 bar. Therefore, at a temperature of 37,8 °C it can only exist as a gas and not as a liquid. | ||
The 1920’s saw the first attempts to cornrnercialise bottled LPG for domestic use. For many years, butanes and propane were transported in pressurised condition in relatively small quantities and were mainly used close to their production points. It was necessary to wait until the 1960’s and the development of the refrigerated ship to see large scale expansion of marine transportation. This, in turn, allowed the recovery of large quantities of LPG available in remote oil production areas.

In 1997 the world-wide production of LPG was 179,6 million tonnes and approximately 47 million tonnes were traded internationally. This quantity is expected to increase to 62 million tonnes by year 2005.
Liquefied natural gas (LNG) – origin and characteristics
Gas Freeing of Cargo Tanks on Liquefied Natural Gas CarriersLiquid Natural Gas (LNG) is made up, for the most part, of Methane (CH4), which accounts for between 75 % and 95 % of its volume. Natural gas is colourless at ambient temperature and it is also odourless and non-toxic.
Natural gas can be obtained from three different sources:
- underground wells, which are mainly gas bearing (non-associated gas);
- condensate reservoirs;
- large oil fields (associated gas).
In the case of associated gas, natural gas may be found either in solution with the crude oil or as a gas cap above it.
Natural gas contains smaller quantities of heavier hydrocarbons, in addition to varying amounts of:
- water;
- carbon dioxide;
- hydrogen sulphide;
- nitrogen and other non-hydrocarbon substances.
The constituents vary, depending on its geographical origin. It can comprise:
| Methane | 75 %-95 % |
| Nitrogen | 5 %-0,2 % |
| Ethane | 1 %-25 % |
| Propane | |
| Butane | |
| Pentane |
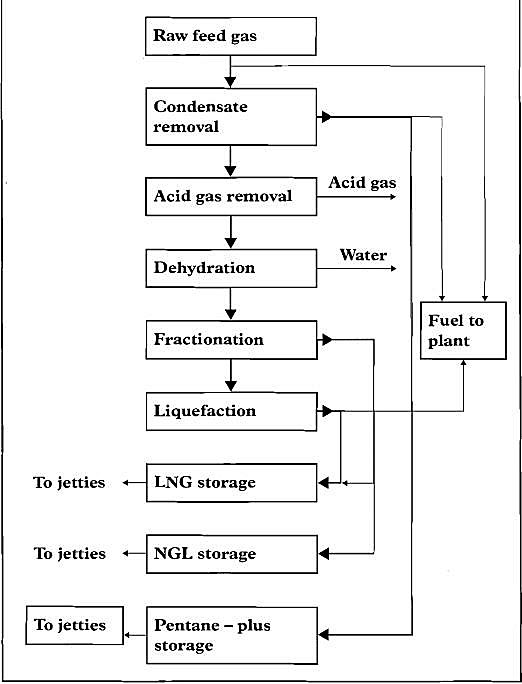
Natural gas is liquefied for shipping at a temperature of approximately -160 °C. By liquefying the gas, its volume is reduced by a factor of 600, which means that LNG at -160 °C uses 1/600 of the space required for the same weight of gas at ambient temperature. Hydrogen sulphide and carbon dioxide must be removed prior to liquefaction. This is generally referred to as gas sweetening.
LNG is a clear liquid, much lighter than water, with a density between 430 and 520 kg/dm3. A mixture of 5 % to 14 % of methane gas in air can ignite when in contact with a spark or naked flame.
When the liquid is loaded onto a ship, it immediately starts to «boil», or return to vapour form as it warms up by cooling the ship’s containment system and from heat leakages through the tank insulation. The lighter constituents, having lower Boiling Point – Definition and Pronunciationboiling points, vaporise first. Nitrogen, although having a higher molecular weight than methane, has a lower boiling point and forms a large part of the initial boil-off gas. The vapour phase of a tank can include up to 50 % or more nitrogen in the initial hours after loading, depending on the composition of the LNG. This is important because, on LNG tankers, boil-off vapour is used as fuel in the ship’s boiler. In this case, the usable combustible gas is reduced by the nitrogen content and the combustion control system must be designed to take this into account. Evaporation at different rates means that the gas delivered at the end of the voyage has a slightly lower proportion of nitrogen and methane than when loaded and a higher proportion of ethane, propane and butane.
When LNG is exposed to ambient temperature, as in the case of a leak, for example, it vaporises quickly. At liquid temperature, the gas is 1,4 times heavier than air, but, as it becomes warmer, its density decreases, reaching 0,55 times that of air at ambient temperature.
Cargo Related Spaces on Liquefied Natural Gas CarriersNatural gas has a calorific value higher than normal oil fuels but lower than LPG‘s, yet it is perceived as «clean». The combustion of natural gas releases lower amounts of soot, nitrogen oxides, sulphur oxides or carbon monoxide than other LPG, oil and solid fuels. The released amount of carbon dioxide is also lower than for other hydrocarbon fuels, at about 0,2 kg/kWh against 0,33 kg/kWh for coal and 0,26 kg/kWh for domestic fuel oil.
In 1997, almost 182 million cubic meters (or 82 million tonnes) of LNG were carried by sea and this is expected to rise to more than 100 million tonnes by 2005.

Liquefied petrochemical gases
General
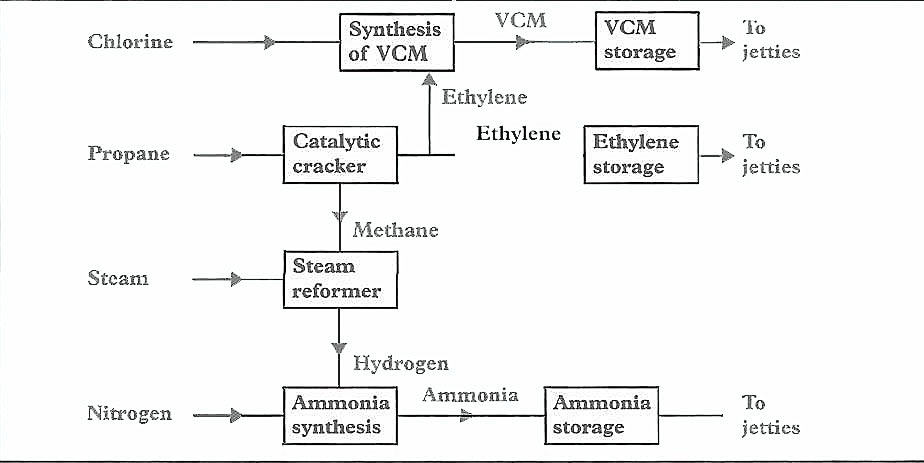
A number of chemical gases or gases allied to or derived from within the Prospects of Liquefied Petroleum Gas Industry and Why it is a Growing Marketpetroleum industry have similar physical properties as LPG and are shipped on LPG carriers. They are commonly referred to as Liquefied Petrochemical Gases and can include ammonia (NH3), butadiene (C4H6), propylene (C3H6), vinyl chloride monomer (VCM) (C2H3Cl) and ethylene (C2H4). Petrochemical gases include other products but these five are the ones principally transported by sea and their origins and characteristics are described below.
Ammonia (NH3)
Ammonia is derived from synthesis gas by catalytic reforming, synthesis gas being produced by reforming water and a hydrocarbon such as coal, natural gas, petroleum gas or naphtha. The type of production process is thus determined by the feedstock available and this, in turn, determines the plant location. Natural gas forms the basis of approximately 80 % of current production capacity.
Ammonia is a colourless gas with a characteristic pungent odour. It is very soluble in water; one volume of water will dissolve about 700 volumes of ammonia at 20 °C. Anhydrous ammonia is readily condensable to liquid at moderate pressures and temperatures and is commonly known as liquid ammonia. In liquid form and at atmospheric pressure, ammonia is lighter than water, with a density of 0,680 kg/dm3. Ammonia can be shipped in pressurised condition at ambient temperature or fully refrigerated at atmospheric pressure at a temperature of -33,4 °C. Ammonia is less flammable than other hydrocarbon gases; a mixture of ammonia gas in air is only flammable in the range of 14 % to 27 %, but it is poisonous and has a sharply irritating effect on the eyes, mucous membranes of the respiratory tract and on the moist areas of the skin. Below 60 °C, Ammonia reacts with CO2, to form ammonium carbamate, a white salt crust which adheres to the tank walls.
Ships carrying ammonia have to take special precautions to avoid any possibility of moisture contamination of the cargo as this can lead to the phenomenon of stress corrosion cracking which can be very damaging to the cargo tanks.
In 1997, world-wide production of ammonia was 120 million tonnes, of which approximately 10 % was internationally traded. This quantity has been stable during recent years.
Butadiene (C4H6)
Butadiene is one of the olefins group and 90 % of production is used by the rubber industry. It is also used in paints and binders for non-woven fabrics and as an intermediary in plastic and nylon production. Butadiene is mainly obtained by extraction from crude C4 streams produced as a by-product of ethylene manufacture and refinery operations. It can also be obtained from the dehydrogenation of n-butane and from ethyl alcohol.
Butadiene is a colourless gas with a strangely aromatic odour. It is slightly poisonous but strongly carcinogenic. In high concentrations, it irritates the skin and acts as an anaesthetic. It is easily condensable to liquid at moderate pressures and temperatures.
In liquid form and at atmospheric pressure, it is lighter than water with a density of 0,650 kg/dm3. Brought into contact with air, butadiene readily forms peroxides, which do not dissolve in liquid butadiene. These peroxides are very sensitive to heat and to impact and can lead to uncontrollable explosions. Heat, short-wave or the presence of peroxides or other agents can polymerise the butadiene, producing, in the liquid phase, a dark rubber-like polymer and, in the gas phase, white crystals. For these reasons, butadiene is mainly shipped in a fully refrigerated condition at atmospheric pressure at a temperature of -5 °C, with the addition of stabilisers to prevent polymerisation. Mixtures of 2 % to 11,5 % of butadiene gas in air can ignite when in contact with a spark or a naked gas flame.
In 1997, world-wide production of butadiene was more than 8 million tonnes, of which approximately 800,000 tonnes were transported by sea.
Propylene (C3H6)
Propylene is also one of the olefins group and is polymerised into polypropylene, which is used in the manufacture of moulded components for:
- cars and domestic appliances;
- carpet fibres;
- cable sheathing;
- piping;
- coatings and containers.
Propylene is mainly produced as a by-product of ethylene (70 %) but it can also be produced by refineries (29 %) or through propane dehydrogenation (1 %), a process which should see a considerable development due to the expected deficit in propylene forecast for the near future.
Propylene is a colourless gas with narcotic properties which burns in air with a yellow soot-forming flame. In high concentrations, it has a slight and strange odour. It is easily condensable to liquid at moderate pressures and temperatures. In liquid form and at atmospheric pressure, it is lighter than water, with a density of 0,612 kg/dm3. Propylene can be shipped in pressurised condition at ambient temperature or fully refrigerated at atmospheric pressure at a temperature of -47,7 °C. Mixtures of 2,2 % to 10,2 % of propylene gas in air can ignite when in contact with a spark or a naked flame.
In 1996, world-wide production of propylene was 41 million tonnes and approximately 2,7 million tonnes were transported by sea.
Vinyl chloride monomer (VCM) (C2H3Cl)
VCM is produced by the oxychlorination process which treats ethylene to produce ethylene dichloride which then gives VCM through pyrolysis or by reaction with caustic soda. It can also be produced from acetylene and hydrogen chloride. VCM is mainly used in the production of plastics (polyvinylchlorides or PVC).
Vinyl Chloride Monomer is a colourless, slightly poisonous gas. In high concentration, it has a pleasant, sweet odour and acts as a narcotic. VCM is a strong carcinogenic agent. It is easily condensable to liquid at moderate pressures and temperatures. In liquid form and at atmospheric pressure, it is almost as heavy as water, with a density of 0,97 kg/dm3. Vinyl chloride decomposes in an open flame and gives phosgenes. VCM can be shipped in pressurised condition at ambient temperature or fully refrigerated at atmospheric pressure at a temperature of – 13,8 °C. Mixtures of 4 % to 29,3 % of VCM gas in air can be inflamed by contact with a spark or a naked flame.
In 1996, world-wide production of VCM was more than 22 million tonnes of which 786,000 tonnes were transported by sea.

Ethylene (C2H4)
Ethylene is one of the most basic components of the petrochemical industry. Its production involves «cracking» a suitable feedstock such as ethane, propane, butane, naphtha or gasoil into ethylene and several other co-products including propylene and butadiene. Ethylene is polymerised into high density polyethylene (HDPE), low density polyethylene (LDPE) and linear low density polyethylene (LLDPE) which, in turn, are used to make plastic packaging, films, bottles, containers and household hardware. Ethylene glycol, used as antifreeze, is produced by oxidation and hydration. Ethylene is used with benzene in the manufacture of styrene to produce polystyrene for insulation, packaging and toys.
Read also: Main Procedures and Best Practices of Liquefied Gas Discharge from LNG Carriers
Ethylene is a colourless, practically odourless and slightly poisonous gas. As an olefin, it is more narcotic than lower paraffin (propane, butane). Ethylene burns in air with a soot-forming red luminous flame. It is condensable to liquid at a very low temperature. In liquid form at atmospheric pressure, it is lighter than water, with a density of 0,570 kg/dm3. Ethylene can be shipped either in fully refrigerated condition at atmospheric pressure at a temperature of -103,9 °C or under a pressure of about 5 bars at a temperature of approximately -75 °C.
| Physical Properties of Gases (SIGTTO) | ||||||
|---|---|---|---|---|---|---|
| Gas | Atmospheric boiling point (°C) | Critical temperature (°С) | Critical pressure (bar, abs.) | Condensing ratio dm3 liquid/m3 gas | Liquid relative density at Atm. boiling pt. (Water = 1) | Vapour relative density (Air = 1) |
| Methane | -161,5 | -82,5 | 44,7 | 0,804 | 0,427 | 0,554 |
| Ethane | -88,6 | 32,1 | 48,9 | 2,453 | 0,54 | 1,048 |
| Propane | -42,3 | 96,8 | 42,6 | 3,38 | 0,583 | 1,55 |
| n-Butane | -0,5 | 153 | 38,1 | 4,32 | 0,6 | 2,09 |
| i -Butane | -11,7 | 133,7 | 38,2 | 4,36 | 0,596 | 2,07 |
| Ethylene | -103,9 | 9,9 | 50,5 | 2,2 | 0,57 | 0,975 |
| Propylene | -47,7 | 92,1 | 45,6 | 3,08 | 0,613 | 1,48 |
| α-Butylene | -6,1 | 146,4 | 38,9 | 4,01 | 0,624 | 1,94 |
| γ-Butylene | -6,9 | 144,7 | 38,7 | 4 | 0,627 | 1,94 |
| Butadiene | -5 | 161,8 | 43,2 | 3,81 | 0,653 | 1,88 |
| Isoprene | 34 | 211 | 38,5 | 0,67 | 2,3 | |
| Vinyl chloride | -13,8 | 158,4 | 52,9 | 2,87 | 0,965 | 2,15 |
| Ethylene oxide | 10,7 | 195,7 | 74,4 | 2,13 | 0,896 | 1,52 |
| Propylene oxide | 34,2 | 209,1 | 47,7 | 0,83 | 2 | |
| Ammonia | -33,4 | 132,4 | 113 | 1,12 | 0,683 | 0,597 |
| Chlorine | -34 | 144 | 77,1 | 2,03 | 1,56 | 2,49 |
Because of this low temperature, it is necessary to have special ships for the transportation of ethylene. A mixture of 2,7 % to 28,5 % of ethylene gas in air can ignite when in contact with a spark or naked flame.
In 1996 the world-wide production of ethylene was more than 75 million tonnes of which approximately 3 million tonnes were transported by sea.

