LNG, or liquefied natural gas, primarily consists of methane (CH4), with small amounts of other hydrocarbons such as ethane (C2H6), propane (C3H8), and butane (C4H10), as well as traces of nitrogen, carbon dioxide, and sulfur compounds. The molecular structure of LNG is characterized by its predominantly single – bonded carbon and hydrogen atoms, forming a tetrahedral geometry around each carbon atom. As a result, LNG exhibits a relatively simple molecular structure compared to other hydrocarbon compounds. In theoretical models, LNG is often treated as an ideal gas, simplifying calculations and predictions regarding its behavior under different conditions.
- Definition of LNG
- Methane
- Definitions of LPG
- Chemistry of Gases
- Definitions and Physical Properties of Gases
- Vapor
- Dryness Fraction
- Critical Temperature
- Critical Pressure
- Atmospheric Boiling Point
- Liquid Gases Specific Gravity
- Mixtures of Liquid Gases
- Isomers
- Ideal Gas Laws
- Latent Heat of Vaporisation
- Enthalpy
- Entropy
- Mollier Diagram
- Hazards of Liquefied Gas Cargoes
- Flammability
- Polymerization
- Health Hazards
- Corrosion
- Hydrates
- Rollover
- Cold
- Summary of Methane Hazards and Emergency Procedures
- Spillage
- Contact with Liquid
- Vapor Inhaled
In the context of an ideal gas, LNG behaves according to the principles of the ideal gas law, which describes the relationship between pressure, volume, temperature, and the number of gas molecules. According to this law, LNG molecules are assumed to occupy negligible volume and exhibit no intermolecular forces, resulting in elastic collisions with the container walls. Consequently, LNG can expand to fill any container, assuming its shape, and its pressure, volume, and temperature are directly related through the equation P V = n R T, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature. This idealized behavior simplifies calculations and predictions related to the Properties of liquified gasesproperties and behavior of LNG.
Definition of LNG
LNG is the common acronym for “Liquefied natural gas“. Natural gas is a mixture of gases that is produced with or without oil in gas and/or oil fields and consists primarily of methane. Liquefaction of methane is achieved by cooling the gas to below minus 160 ˚C under normal atmospheric pressure. Natural gas in liquid form is some 600 – 620 times less in volume than its gaseous equivalent.
The actual percentage of methane in the natural gas depends on the characteristics of the oil field where it is produced. Table 1 indicates typical composition of natural gases depending on the area of production.
| Table 1. Composition of natural gases | ||||||||
|---|---|---|---|---|---|---|---|---|
| Components | Production Area | |||||||
| Abu Dhabi | Alaska | Algeria | Brunei | Iran | Lybia | North Sea | NW Shelf | |
| Methane | 90,37 % | 99,5 % | 86,30 % | 88,00 % | 96,30 % | 66,80 % | 85,90 % | 89,90 % |
| Ethane | 7,01 % | 0,10 % | 7,80 % | 5,10 % | 1,20 % | 19,40 % | 8,10 % | 6,70 % |
| Propane | 1,32 % | 3,20 % | 4,80 % | 0,40 % | 9,10 % | 2,70 % | 2,30 % | |
| Butane | 0,55 % | 0,60 % | 1,80 % | 0,20 % | 3,50 % | 0,90 % | 1,00 % | |
| Pentane | 0,10 % | 0,20 % | 0,10 % | 1,20 % | 0,30 % | 0,04 % | ||
| Nitrogen | 0,75 % | 0,40 % | 0,10 % | 1,30 % | 0,50 % | 0,08 % | ||
| CO2 | 1,00 % | |||||||
Note that, in general, the process of liquefaction of the natural gas increases the percentage of methane. That means that the average composition of a natural gas coming from one of the countries indicated in Table 1, does not reflect the actual composition of the LNG coming from that particular natural gas. Also, note that the LNG is not to be evaluated on basis of the percentage of methane: in other words, the equation: “greater percentage of methane = higher quality LNG” is not necessarily true.
Methane
Methane is colorless and odorless both in liquid and gaseous states. The followings are its main physical characteristics:
- Chemical composition: CH4.
- Boiling point at atmospheric pressure: -161,5 °C.
- Specific gravity of liquid at -160 ˚C: 458 kg/m3.
- Specific gravity of gas at 30 ˚C: 0,67 kg/m3.
- Critical pressure: 44,7 bar.
- Critical temperature: -82,5 °C.
- Heat of vaporization at boiling point: 121 kcal/kg.
- Lower flammability point in air: 5,3 %.
- Upper flammability point in air: 14 %.
- Flash point: -175 °C.
- Spontaneous ignition temperature: 595 °C.
- Minimum ignition energy of flammable mixture in air: 1 mill joule.
The very high percentage of methane in all the natural gases may permit us to assume the above characteristics as valid for natural gases, at least as a first approximation. As already mentioned, in general LNG has a higher methane content percentage than the natural gas as produced at the well.
Definitions of LPG
LPG refers to “Liquefied Petroleum Gas“. LPG’s main components are butane and propane. LPG’s have higher boiling points than methane and liquefy at temperatures between -5 and -45 ˚C at atmospheric pressure.
As indicated by the name, general petroleum gases are refined during the oil refinery process. However, small percentages of petroleum gases are also contained in the natural gases.
While the term LPG should apply in particular to butane and propane, in general it is broadly used to indicate all the gases contemplated by the IMO Gas Code, including chemical gases but excluding LNG (Methane) and refrigerating gases. In particular, a LPG carrier is a ship that can carry not only LPG but also chemical gases, as permitted by its design and by the IMO Gas Code.
The physical properties of the LPG, in particular the specific gravity, boiling point at atmospheric pressure, critical temperature and critical pressure are quite variable depending on the particular gas considered. These differences lead to differences of the most convenient conditions (in term of temperature and pressure) for their transportation and in difference of the design of the tanks (and as a consequence of the ship) used for their storage and transportation.
Chemistry of Gases
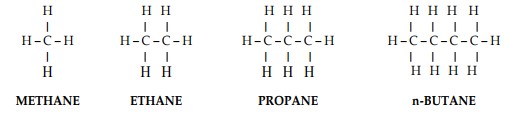
Methane, Ethane, Propane, etc. are hydrocarbons. Hydrocarbons are substances whose molecules contain only hydrogen and carbon atoms. The carbon atom has four bonds and the hydrogen atom has one bond. The combinations that can be obtained combining the bonds of carbon and hydrogen are almost infinite. They start from a very simple combination to combinations more and more complicated. In particular, when the combinations are such as each one of the four carbon bonds combines with one bond of another atom, the hydrocarbon is defined saturated. The saturated hydrocarbons are more stable than the other (unsaturated) hydrocarbons. The simplest existing saturated hydrocarbon is methane, whose molecule is composed by one atom of carbon and four atoms of hydrogen.
The following formulas show (Figure 1) the constitution of the molecule of the first simplest saturated hydrocarbons.
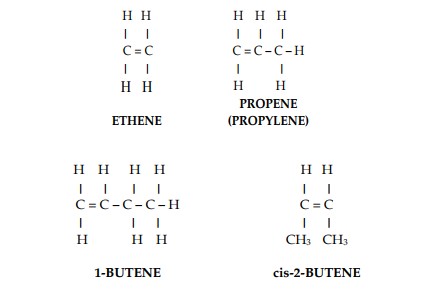
Where there is less than the full complement of hydrogen atoms, two or more carbon atoms are linked by double or triple bonds. These links are weaker than the single bond links and for this reasons these hydrocarbons are called unsaturated hydrocarbons (Figure 2).
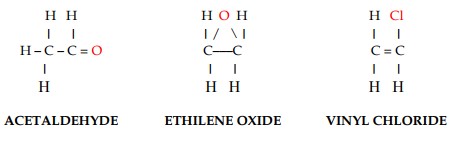
A third group of liquefied gases is constituted by chemical gases. These gases are characterized by the addition of atoms other than carbon and hydrogen to the normal structure of the hydrocarbons (either saturated or unsaturated). The following are three examples of chemical gases (Figure 3).
Definitions and Physical Properties of Gases
Vapor
Vapor is the name normally given to a gas at a temperature below its critical temperature. In particular conditions, vapor may exist in equilibrium with its liquid. Where vapor is contained in a tank, the boundaries of the tank are pressurized.
In the case vapor is in stable equilibrium with its liquid, the vapor is saturated: the pressure of the saturated vapor depends only on its temperature. Vapor is superheated when its temperature is above the temperature corresponding to saturation conditions.
The pressure of a superheated vapor depends on the volume.
Water vapor is generally indicated as steam.
Dryness Fraction
Dryness fraction of a mixture of saturated vapor and its liquid is the ratio between the vapor mass and the mixture mass. For this reason the dryness fraction has no physical dimension. The dryness fraction may vary between 0 (saturated liquid without vapor) to 1 (dry saturated vapor). All intermediate figures are relative to humid saturated vapor; the word humid meaning that the saturated vapor is in equilibrium with a certain quantity of its liquid.
Critical Temperature
The critical temperature of a gas is the temperature above which it cannot be liquefied, no matter how great is the applied pressure. This temperature is important as it is the main factor to be taken into account when it is decided to load the gas in refrigerated or pressurized (or any combination of both) tanks and accordingly it is the main factor for the design of a gas carrier ship. Table 2 indicates the critical temperatures of all the LPG and chemical gases listed in IMO Gas Code as well as those of methane and water for reference.
| Table 2. Physical characteristics of gases | ||||
|---|---|---|---|---|
| SUBSTANCE | CRITICAL TEMPERATURE (°C) | CRITICAL PRESSURE (bar) | ATMOSPHERIC BOILING POINT (°C) | LIQUID DENSITY (kg/m3) |
| METHANE | – 82,5 | 44,7 | 161,5 | 458 |
| Ammonia | +132,4 | 113,0 | -33,4 | 683 |
| Butadiene | +161,8 | 43,2 | -5,0 | 653 |
| i-Butane | +133,7 | 38,2r | -11,7 | 596 |
| n-Butane | +153,0 | 38,1 | -0,5 | 600 |
| A-Butylenes | +146,4 | 38,9 | -6,1 | 624 |
| Г-Butylenes | +144,7 | 38,7 | -6,9 | 627 |
| Chlorine | +144,0 | 77,1 | -34,0 | 1560 |
| Ethane | +32,1 | 48,9 | -88,6 | 540 |
| Ethylene | +9,9 | 50,5 | -103,9 | 570 |
| Ethylene oxide | +195,7 | 74,4 | +10,73 | 896 |
| Isoprene | +211,0 | 38,5 | +34,0 | 670 |
| Propane | +96,8 | 42,6 | -42,3 | 583 |
| Propylene | +92,1 | 45,6 | -47,7 | 613 |
| Propylene oxide | +209,1 | 47,7 | +34,2 | 830 |
| Vinyl chloride | +158,4 | 52,9 | -13,8 | 965 |
| WATER | +374,1 | 22,1 | +100,0 | 1000 |
Critical Pressure
The critical pressure of a gas is the pressure required to liquefy a gas at its critical temperature by pressurization. Also this pressure in association with the critical temperature is important to decide how the liquefied gas is to be loaded in a tank. Table 2 indicates the critical pressures of the LPG and chemical gases listed in IMO Gas Code, compared with the critical pressures of methane and water for reference.
Atmospheric Boiling Point
The couple “critical temperature and critical pressure” is the extreme couple of Gas laws, thermodynamic principles and reliquefactiontemperature and pressure values at, which a change of status from liquid to vapor or from vapor to liquid may happen. However, there are infinite couples of temperature and pressures at, which a gas may be liquefied. For each substance to be transported, the most convenient couple is selected. In general, the most convenient couple is the one making reference to the atmospheric pressure. Higher values of pressure, in fact, would require pressure vessels for transportation of liquid gases. It is therefore important to know the liquefaction temperature of the gases at the atmospheric pressure (the atmospheric boiling point). Table 2 indicates these temperatures for the LPG and chemical gases listed in IMO Gas Code compared with those of methane and water for reference.
Taking into account the critical temperature and pressure of each gas, as well as its atmospheric boiling point, it appears that the majority of the gases listed in Table 2 may be liquefied by pressurization only, maintaining a temperature in the range of the ambient temperatures at reasonable acceptable pressure. This is not valid for methane, ethane and ethylene. If methane were cooled at least up to its critical temperature, its critical pressure would be so high as not to permit the construction of acceptable containers, especially considering the large size of the LNG carrier tanks. This leads to the necessity to load LNG at a temperature below its atmospheric boiling point allowing the use of non-pressurized (or at least very slightly pressurized) tanks, only subjected to the static loads due to the cargo’s own weight and to the dynamic loads (sloshing) due to ship and cargo movements.
Liquid Gases Specific Gravity
For the design of the cargo containment system, it is important to know the weight of the cargoes in the tanks. Table 2 indicates the liquid gas specific gravity of the LPG’s and chemical gases listed in IMO IGC Code code compared with those of methane and water for reference.
It is to be noted that while liquid methane specific gravity is about one half of the water specific gravity, methane vapor at low temperatures is heavier than air. Increasing the temperature of methane, its vapor becomes lighter and at the temperature of about minus 100 °C it has the same weight of air. At higher temperature methane vapor becomes lighter than air. Figure 4 indicates the pattern of the density ratio between methane vapor and air.
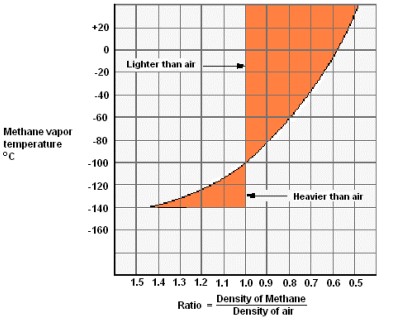
Mixtures of Liquid Gases
It is to be noted that in a mixture of liquefied gases, all the components are dissolved in each other’s. This is why components having lower boiling points than others remain liquid even though the temperature of the mixture reaches higher values than their own boiling points.
Isomers
The physical properties of the gases depend on their molecular structure. Some substances have the same molecular formula, but a different atom arrangement. Two substances with molecules composed by the same number and type of atoms, but with a different structureare called isomers. The isomers have different physical properties even though have the same chemical molecular formula.
Ideal Gas Laws
A gas would be categorized as an “Ideal Gas“, if it were able to comply with the theoretical laws of the ideal gases. Although an ideal gas does not exist, the ideal gas laws are approached quite well by most gases and may be used as a first approximation for practical purposes, in particular, for non-saturated gases at ambient temperatures and moderate pressures.
Ideal gas laws establish a number of relations between the absolute pressure, the absolute temperature and the volume of a certain amount of ideal gas. Of course, in the following formulae compatible units are to be used.
a) Boyle’s law
It states that, at a constant temperature, the volume of a fixed mass of gas varies inversely with its absolute pressure:
or
.
b) Charle’s law
It states that, at a constant pressure, the volume of a fixed mass of gas varies directly with its absolute temperature:
or
c) Pressure law
It states that, at constant volume, the pressure of a fixed mass of gas varies directly with its absolute temperature:
or
d) General Ideal Gas law
The above three laws may be combined into the following:
or, more generally, using the Universal Ideal Gas Constant:
e) Raoult’s law
It states that the total pressure of a mixture of n gases in a certain volume is equal to the sum of the partial pressures of each component if it was contained alone in that volume.
where:
- P, P1 and P2 – are absolute pressures.
- V, V1 and V2 – are volumes.
- T, T1 and T2 – are temperatures.
- M – is the mass of the gas.
- m – is the molecular weight.
- R – is the Universal Ideal Gas Constant = 8,314 kJ / kg mol K.
- P*1, P*2, P*3, xnP*n – is the pressure that would have been created by pure component 1, 2, 3, n at the temperature of the mixture.
- x1, x2, x3, xn – is the mole Mole is the quantity of substance containing a number of particles (atoms, ions, molecules) equal to the number of atoms contained in 12 grams of carbon 12. By definition, one mole of any substance always contains the same number of particles: this number is known as the Avogadro’s number and it is equal to 6,023 × 1023. For instance, considering that one carbon atom has mass about 12 times greater than an atom of hydrogen, this means that 12 g of carbon and 1 g of hydrogen contain the same number of particles.x fraction of component 1, 2, 3, n. This is the percentage of each component.
Latent Heat of Vaporisation
Many substances may exist in solid, liquid or vapor state. In order to change its state from solid to liquid and from liquid to vapor, heat must be given to a substance. Similarly, in order to change its state from liquid to solid and from vapor to liquid, the substance must give heat. The heat given or to be given by a substance to change its state is called latent heat. For transportation of liquid gases the latent heat relative to the change of status from liquid to solid or from solid to liquid is of no interest, as the values of temperature and pressures necessary for the solidification are so far from the range of values applied to the transportation of gases by sea that there does not exist any possibility of solidification of cargo.
When cold liquid cargoes are transported, a certain amount of heat is transmitted to the cargo from the external environment through the boundaries of the cargo containment system. No perfect insulation, which would also be commercially available, exists at present time to inhibit all heat transfer / losses through the boundaries of the cargo tanks. Therefore, a certain amount of cargo will vaporize continuously. This generated vapor cannot be left in the tank as it would increase the pressure and thus transform the tank into a pressure vessel. For this reason the generated vapor is led out of the tank to be either disposed of, by using as a fuel in ship’s propulsion / machinery room or refrigerated again by a reliquifaction plant, generally installed on the deck of the cargo area of the gas carriers, and then sent back to the cargo tanks as a liquid.
Enthalpy
Enthalpy is the thermodynamic measure of the total heat content of a liquid or vapor at a given temperature.
The enthalpy of a certain mass of substance is calculated with the following formula:
where:
- H – is the enthalpy (kJ/kg).
- U – is the internal energy (kJ/kg).
- P – is the absolute pressure (kN/m2).
- V – is the total volume of the system liquid plus vapor (m3).
- M – is the mass of the system (kg).
A variation of enthalpy through an isentropic (at constant entropy) thermodynamic process is equal to the external work done by the system during the process.
Since a variation of enthalpy represents the total energy change in a fluid as it passes through any thermodynamic process and the transformations of fluid status in the thermal machines may be considered isentropic, enthalpy is a useful unit for the analysis of energy changes, in particular for the cyclic processes involving evaporation, compression, condensation and expansion, such as the reliquefaction cycle of the vapor generated in the cargo tanks (boil-off) of the refrigerated LPG carriers.
Entropy
Entropy is a thermodynamic quantity used in the study of processes and can be visualized as the degree of disorder of system. It is most useful to consider the change in entropy in a process rather than the absolute entropy values. The change in entropy in a reversible process is given by the amount of heat rejected or absorbed by a system divided by the absolute temperature, at, which the heat exchange occurs. In an adiabatic process (in, which there is no heat rejected or absorbed) the change of entropy is zero.
where:
- ΔS = entropy variation;
- ΔQ = occurred heat exchange;
- T = temperature at, which the exchanged occurred.
Mollier Diagram
The Mollier diagram is the most important tool to study the thermodynamic processes where involving change of status of fluids, such as those in steam turbines, refrigerating machines, etc. Figure 5 shows a simplified example of Mollier diagram for water and steam. Similar diagrams may be prepared for any other vapor.
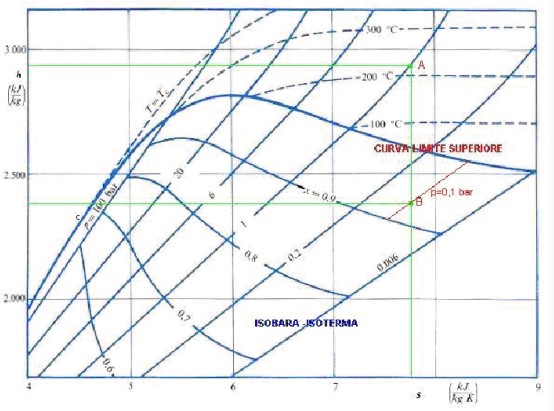
The diagram indicates in the horizontal axis the entropy of the vapor and in the vertical axis its enthalpy. On the diagram the followings are indicated:
- The curves of equal values of the dryness fraction. These curves have a bell shape and are in the lower part of the diagram under the top bell shaped curve, which is indicated as the limit curve. The limit curve corresponds with the dryness fraction 1 and therefore it divides the diagram into two sections. The upper section refers to dry superheated vapor, the lower section to humid saturated vapor.
- The isotherm curves (the curves indicating equal values of temperature). They are above the limit curve in the upper part of the diagram. These curves, which have growing values towards the high part of the diagram, are directed towards the left side of the diagram and have the trend to become almost horizontal.
- The isobar curves (the curves indicating equal values of the pressure). They are above the limit curve in the upper part of the diagram. These curves, which have growing values towards the left part of the diagram, are directed towards the high part of the diagram and trend to become almost vertical.
- The isobar – isotherm curves. They are below the limit curve in the lower part of the diagram. These curves, which have growing values towards the left part of the diagram, are straight and are inclined toward the left part of the diagram. When they encounter the limit curve, they continue above it as the isobar curves described above.
- The equal volume curves are often also indicated in the diagram. They have not been indicated in the example so as not to crowd the other lines. Their pattern is similar to that of the isobar curves, however they are steeper.
Once the point representing the initial conditions of the vapor has been established in the diagram at the intersection of the curves indicating its pressure and its dryness fraction (or temperature), it is possible to trace a vertical line from this point to the intersection with the curve representing the final pressure of the vapor after the transformation. The length of the vertical line represents the reduction of enthalpy occurring in an adiabatic expansion. That means that it corresponds to the work done by 1 kg of vapor in a turbine (not considering passive losses).
In the example of Figure 5, the initial state of the superheated steam (point A) is: pressure = 1 bar, temperature = 220 °C. If we consider an adiabatic expansion up to a pressure of 0,1 bar (or 0,93 dryness fraction) the point B is individuated. The vertical distance AB is the reduction of enthalpy and therefore (not considering the losses) is the work done by the expansion: in this case each kg of steam does a work of about 550 kJ.
In the same way, drawing a liquefaction cycle on the appropriate Mollier diagram, it is possible to evaluate the energetic exchanges during a reliquifaction cycle.
Hazards of Liquefied Gas Cargoes
The following is the list of all Hazards of LNG and Relevant Gasestypes of hazards, which may be encountered handling and transporting liquid gases. For each hazard there will be the indication whether this hazard is likely for LNG, for one or more LPG’s or for both.
Flammability
Flammability is a hazard that is common to LNG and all LPG excluding chlorine. In order to evaluate the risk of flammability, the following definitions and characteristics are to be taken into account:
a) COMBUSTION
Combustion is the chemical reaction of oxidation. During the reaction, which is initiated by a source of ignition, a flammable vapor combines with the oxygen contained in the atmosphere producing heat, carbon dioxide and water vapor.
b) FLAMMABLE RANGE
Flammable range is the range included between the minimum and the maximum concentration of a vapor in air (percentage of the volume), which forms a flammable mixture. The lower limit is an indication of the minimum quantity of vapor necessary to start a combustion, it is in general abbreviated to LFL (lower flammability limit): the upper limit, which is in general abbreviated to UFL (upper flammability limit), gives an indication of the maximum concentration of vapor at which combustion will take place. The flammable range varies depending on the gases: typical values of LFL and UFL for certain gases are as indicated below:
- Methane 5,3 % – 14 %.
- Propane 2,1 % – 9,5 %.
- Ethylene 3,0 % – 34,0 %.
- Ethylene oxide 3,0 % – 100 %.
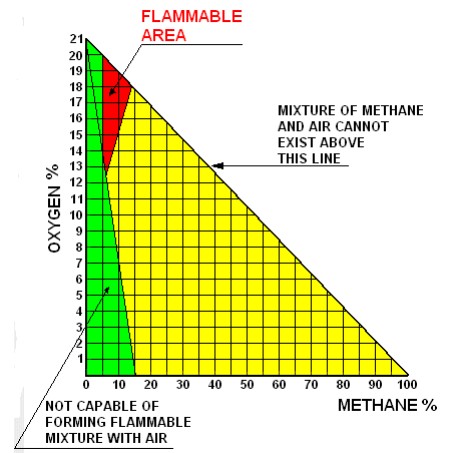
The above values are based on an atmosphere with oxygen content of 21 %, of course if the content of oxygen in the atmosphere is reduced, also the above values will change. Figure 6 gives the flammable range of methane for all possible concentrations of oxygen in the atmosphere (from 0 % to 21 %).
c) FLASH POINT
Flash Point – Definition and PronunciationFlash point of a liquid is the lowest temperature at which a liquid generates enough vapor to create a flammable mixture with air. This temperature is quite low, for instance -175 °C for methane and -105 °C for propane. Even though the liquid gases are transported at temperature above their flash points, the atmosphere inside the tank is not flammable as it is completed saturated by the cargo vapor, well above the UFL.
d) AUTO-IGNITION TEMPERATURE
Auto-ignition temperature of a substance is that temperature at, which a flammable mixture vapor – air ignites and starts the combustion spontaneously. It is important to know the auto-ignition temperature when there is the risk of presence of flammable mixture in correspondence of hot surfaces. Typical values of auto-ignition temperature are:
- Methane 595 °C.
- Isoprene 220 °C.
- Acetaldehyde 165 °C.
e) MINIMUM IGNITION ENERGY
Minimum ignition energy is the energy that is necessary to start the combustion of a flammable mixture. The minimum ignition energy of a flammable methane atmosphere is less than 1 mill joule: an energy level, which is substantially exceeded by any visible flame (cigarette) power circuit breaker switch-on or switch-off, lights, or electrostatic discharges down the lowest level detectable by human contact.
Polymerization
Some substances are self – reactive and liable to polymerize unless protected by an inhibitor.
Polymerization is a process whereby the molecules of substance link together into new compounds composed by a minimum of two up to thousands of the previous molecules, this new compound is called polymer. The polymerization reaction develops a great deal of heat and therefore can be dangerous. The process can be initiated and accelerated by catalytic factors, such as heat, rust, light, presence of acids or other particular substances, etc. This kind of risk does not involve methane.
Read also: Flammability, Explosion and other Hazards of Liquefied Gas
The following formulas show the process of polymerization of the vinyl chloride (Figure 7).
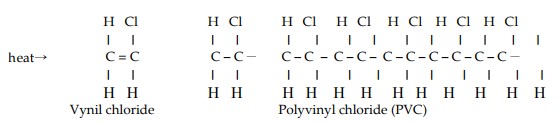
Health Hazards
a) TOXICITY
Some gases are toxic because their chemical properties may cause temporary of permanent damage to the health. The effect may be caused by skin contact, skin wound contact or inhalation. The damages may be irritation, tissue damages or impairment of mental or physical faculties. The maximum concentration of gases, vapors, mist or spray to, which a person may be exposed, day after day, assuming 8 hours per day or 40 hours per week, without adverse consequence is referred to as the Threshold Limit Value (TLV). Methane is a non-toxic gas.
b) ASPHYXIA
Asphyxia may occur when the necessary supply of oxygen for the human being is not available, due to a vapor/gas concentration so high as to reduce the amount of oxygen in the atmosphere. Asphyxia does not depend on the toxicity of the gas. For instance CO2 is a nontoxic gas, but it is extremely dangerous as, being heavier than the air is likely to collect in the lower part of a space where a man can breathe.
c) ANAESTHESIA
A person inhaling certain vapors (for instance ethylene oxide) may lose consciousness due to their effects on the nervous system. An anaesthetized person is unable to perform any activity.
d) FROSTBITE
Direct contact with very cold liquids, vapors, non-insulated pipes or equipment, which are in contact with low temperature fluids, can cause cold burns “frostbite“, which may permanently damage certain organs, such as for instance lungs and eyes.
Corrosion
This hazard is not associated with the transportation of methane. It is a problem more connected to the transportation of chemical gases, in particular anhydrous ammonia and vinyl chloride monomers. Precautions to be taken for the design of the cargo containment and handling systems, when these gasses are transported are indicated in the IGC code.
Hydrates
Propane and butane may form hydrates under certain conditions of pressure and volume in presence of water or other impurity. The hydrates are white crystalline solids that may block the pumps and the valves.
Rollover
In tanks containing fluids of different density, it can happen that in certain conditions, the fluid having greater density (heavier) is in equilibrium over the fluid having minor density (lighter). When this unstable equilibrium is disturbed, the two fluids start to mix with a violent reaction that generates heat. This phenomenon, which is called rollover, is more likely to occur in the on-shore LNG tanks, than in the LNG ships. In fact, it can be provoked by filing a tank still containing some old LNG with some other LNG having different density, or by a change of density of the upper layers due to evaporation, when the LNG is stored for long time.
Cold
This is one of the main hazards connected with the transportation of LNG. At very low temperatures, in fact, the normal steels used for ship construction become very fragile and subject to brittle fracture, similarly to a frozen glass where suddenly hot water is poured.
Summary of Methane Hazards and Emergency Procedures
The following is a list of the possible hazards on LNG ships due to the transportation of LNG and of the emergency procedures should be taken in the case one of these undesirable events should happen.
Spillage
Spillage of LNG may lead to the following dangers:
a) Fire starting from the vapor, which is generated during the spillage.
b) Brittle fractures of the ship structures, which are in contact with the spilled LNG.
c) Brittle fractures of the ship structures, which are in contact with the spilled LNG.
The first two dangers a) and b) will be dealt with more in detail in the following modules. However, should a spillage be detected on a LNG ship the measures to be taken are the following:
- Stop the flow.
- Avoid contacts with liquid and vapor.
- Extinguish all possible sources of ignition.
- Flood the area where the spillage happened with a large amount of water in order to disperse the spilled LNG and to prevent the risk of brittle fracture.
Contact with Liquid
In the case the liquid comes in contact with the skin or the eyes, the consequence is frostbite of the wounded part.
In this event medical assistance is necessary. However, before the intervention of the doctor the following immediate measures are to be taken.
- LIQUID ON SKIN: Affected clothing is to be removed. The frostbitten (wounded) part is to be immersed in warm water until thawed.
- LIQUID ON EYES: Eyes are to be gently flushed with clean fresh/sea water for at least 15 minutes. Following that, the eyes are to be forced open if necessary.
Vapor Inhaled
Inhalation of LNG vapor can provoke asphyxiation, headache, dizziness, and drowsiness (LNG is not poisonous or toxic). If the vapor is cold, the same effects of contact with liquids may happen, moreover lungs may be damaged.
If someone breathes LNG vapor medical assistance is necessary. However, the following immediate measures are to be taken before the intervention of the doctor:
- The injured person is to be removed to fresh air.
- If breathing has stopped, is weak or irregular, mouth to mouth/nose resuscitation is to be given.
