Gas tanker safety culture is crucial to ensuring the well-being of the crew, protecting the environment, and preventing accidents. It involves creating a work environment where safety is prioritized, and all crew members are actively involved in promoting and maintaining safe practices.
- Basic Knowledge of the Hazards
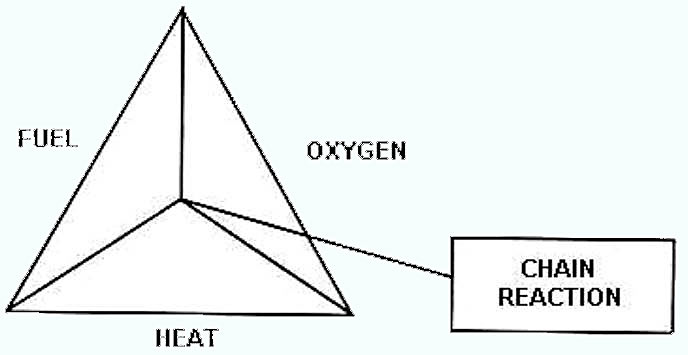
- Explosion & flammability hazards. Triangle of fire/theory
- Heat Transmission
- Classification of fire
- Principles of fire figthing (Extinguishment)
- Health hazard
- Skin contact
- Ingestion
- Inhalation
- Oxygen dificiency
- Inert gas
- Harmful properties of cargoes
- Benzene and Other Aromatic Hydrocarbons
- Hydrogen Sulfide
- First aid do’s and don’t’s
- Toxicity
- Hazard to marine environment
- Reactivity hazards
- Self-reaction and reaction with air
- General precautions for self-reactive cargoes and cargoes which react dangerously with air
- Reactivity with other cargoes
- Reaction with water
- Tank cleaning with water
- Preventing reactivity
- Corrosion hazard
Safety culture on gas tankers includes regular safety training, drills, communication, reporting near-misses, and adhering to safety protocols and procedures. Continuous improvement and a proactive approach to safety are key elements of a strong safety culture on gas tankers.
Masters, officers and ratings appointed to work on tankers or similar vessels must meet the minimum training and qualifications requirements specified in regulation V/1 of the International Conventions on Standards of Training, Certification and Watchkeeping for Seafarers, 1978, as amended in 1995 & 2010.
Training in emergency procedures and in the use of any special emergency equipment should be given as appropriate to members of the crew at regular inter vals. The instruction should include personal first aid measures for dealing with accidental contact with harmful substances in the cargo being carried and inhalation of Common Hazards and Risk Assessment in Oil and Gas Industrydangerous gases and fumes.
Because of the risks of ill effects arising from contamination by certain liquid cargoes, especially those carried in chemical tankers and gas carriers, personnel should maintain very high standards of personal cleanliness and particularly so when they have been engaged in cargo handling and tank cleaning.
Those on board responsible for the safe loading and carriage of the cargo should have all the relevant information about its nature and character before it is loaded and about the precautions which need to be observed during the voyage. The remainder of the crew should be advised of any precautions which they too should observe.
High risks require the strict observance of rules restricting smoking and the carriage of matches or cigarette lighters.
Spillages and leakages of cargo should be attended to promptly.
Oil-soaked rags should not be discarded carelessly where they may be a fire hazard or possibly ignite spontaneously. Other combustible rubbish should not be allowed to accumulate.
Cargo handling equipment, testing instruments, automatic and other alarm systems should be maintained to a very high standard of efficiency at all times. Where electrical equipment is to be used in the cargo area it should be of approved design and «certified safe». The safety of this equipment depends on maintenance of a high order which should be carried out only by competent persons. Unauthorised personnel should not interfere with such equipment. Any faults observed, such as loose or missing fastenings or covers, severe corrosion, cracked or broken lamp, glasses etc should be reported immediately.
Work about the ship which might cause sparking or which involves heat should not be under taken unless authorised after the work area has been tested and found gas-free, or its safety is otherwise assured.
Where any enclosed space has to be entered, the precautions given in Chapter 17 should be strictly observed. Dangerous gases may be released or leak from adjoining spaces while work is in progress and frequent testing of the atmosphere should be under taken.
Liquefied gas carriers
Guidance on the general precautions which should be taken on these vessels is given in the Tanker Safety Guide (Liquefied Gas) and Safety in Liquefied Gas Tankers (a handbook for crew members) published by the International Chamber of Shipping. The IMO Codes for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk contain guidance on operational aspects and are mandator y under the relevant Merchant Shipping regulations.
It should be noted that cargo pipes, valves and connections and any point of leakage at the gas cargo may be intensely cold. Contact may cause severe cold burns.
Pressure should be carefully reduced and liquid cargo drained from any point of the cargo transfer system, including discharge lines, before any opening up or disconnecting is begun.
Some cargoes such as ammonia have a very pungent, suffocating odour and very small quantities may cause eye irritation and disorientation together with chemical burns. Seafarers should take this into account when moving about the vessel, and especially when climbing ladders and gangways. The means of access to the vessel should be such that it can be closely super vised and is sited as far away from the manifold area as possible. Crew members should be aware of the location of eye wash equipment and safety showers.
Basic Knowledge of the Hazards
Explosion & flammability hazards. Triangle of fire/theory
How fire burns: Fire occurs whenever a given material is in the presence of oxygen to a temperature corresponding to the kindling or ignition point of the material. Thus there are three factors, all of which must be present, if fire is to exist. These known as the chemical triangle of fire, the three sides of which are Fuel, Oxygen and Heat Temperature. When all of these three elements are present and brought together in correct proportion, fire burns. Study in fire-fighting starts with this simple fact, because control and extinguishments of fire in general is brought about by removal of these essential elements.
1 Fuel – Any substances that will burn when heated or hot enough in the presence of sufficient oxygen.
2 Oxygen – Comes form the atmosphere we breathe. Atmosphere consist of 21 % oxygen, 78 % and 1 % of other elements. At 15 % of oxygen or below fire will subside. Exception: Alcohol 11 % oxygen smouldering wood 6 %.
3 Heat/Ignition
Heat Temperature (Three Stages)
1 Flash Point – Lowest temperature at which a flammable substance will give off vapour that will ignite when a flame or spark is introduced in the presence of sufficient oxygen.
2 Fire Point – A temperature which is higher than a Flash Point – Definition and Pronunciationflash point at which a substance is heated to give off vapour that will burn without the application of spark or a flame in the presence of sufficient oxygen.
3 Ignition temperature (auto ignition) – Is a temperature which is higher than a fire point at which a substance is heated to give off vapour that will burn without the application of spark or a flame in the presence of sufficient oxygen.
Flammability
The ability of hydrocarbon gases to react with oxygen in the air to produced carbon dioxide and water. This reactions gives enough heat to form a visible flame which travel through the mixture of hydrocarbon gas and air.
Flammability Limit
The limit in which hydrocarbon gas and air cannot ignite and burn unless it composition lies with in a range of gas in air concentration in which there is sufficient hydrocarbon gas to support and propagate combustion.
Upper Flammable Limit (UFL) – The upper limit range of flammability.
Lower Flammable Limit (LFL) -The lower limit range of flammability.
Heat Transmission
Conduction
Transfer of heat through solid bodies. Examples stove, heat is conducted through the pot of its content.
Convection
Transmission of heat through the motion of heated matter, through the motion of smoke, hot air gases, produced by the fire and flying embers.
Radiation
Transmission of heat through atmosphere or transfer of heat from a source across an intervening space. No material substance involved. The heat travels outward from the fire in the same manner as light that is in straight lines.
Sources of ignition
- Direct Heat.
- Mechanical Energy.
- Chemical Energy.
- Electrical Energy.
- Static Electricity.
- Auto Ignition.
- Lightning.
Classification of fire
Class «A» Fires
Fire involving common combustible material.
- Characteristics – deep seated, leaves ashes and embers.
- Extinguishing Agent-Are those that cool off flammable substance.
Class «B» Fire
Fire involving flammable or combustible liquids. Flammable gases, greases and similar products.
- Characteristics – Surface burning.
- Extinguishing Agent – Are those that cut of oxygen supply or prevent flammable vapour from being given off.
Class «C» Fire
Fires involving energized electrical equipment, conductors or appliances.
- Characteristics – Create electrical shock.
- Extinguishing agents – non-conducting extinguishing agents.
Class «D» Fire
Fire involving combustible metals, e. g. magnesium, thermite and sodium.
- Characteristics – Sometimes create violent reaction when the law of nature is applied in extinguishing.
- Extinguishing agents – Depends on the material involved.
Examples:
Magnesium
- Light silvery metal usually alloyed with aluminium.
- Used on aircraft wheels and frames.
- Agents used are dry powder and sand.
- CO2 has no effect. Water fog is also effective, increases the burning rate.
- Cools surrounding area. Solid Stream – creates violent reaction, breaks down.
- Magnesium into oxygen and hydrogen.
Thermite
- 73 % iron oxide, 27 % powdered aluminium, first used in Germany to weld steel.
- No known extinguishing agent.
- It creates own oxygen when burning.
- Best thing to do is jettison.
- Last resort – keep it with solid stream until burned out and at the same time cool the surrounding area.
Sodium
- Soft metallic substance silvery white in colour which oxidizes rapidly in the air.
- Used in bombs and OBA canister.
- Extinguishing Agent – dry powder.
- Do not water, it create violent reaction.
Class «E» Fire
Fire involving Liquefied Gas or Gas Fires.
- Characteristics – Pressure Fires.
- Extinguishing agents – Are those that cut off oxygen supply.
Principles of fire figthing (Extinguishment)
Fire may be extinguished by removing any side of the fire triangle or separation of the fire components.
Successful fire fighting depends on putting into practice one or more of the following principles:
a Cooling to reduce the temperature of a point at which combustion ceases;
Reduction of Heat (Cooling)
- Used something that will absorb heat.
- Water is the best cooling agent.
- Foam contains 94 % water.
b Smothering to reduce the emission of vapours, so that the atmosphere in the vicinity of the fire is below the flammability limit;
Control Oxygen (Smothering)
- Secure the compartment.
- Displacement or diluting the oxygen by means of CO2.
- Blanketing or excluding oxygen by means of foam.
c Replacing the atmosphere in the vicinity of the fire with one containing insufficient oxygen to support combustion;
d Cutting of the supply of combustible material;
Removal of fuel (Starvation)
- Shut of fuel supply.
- Jettisoning.
- Relocate flammable materials away form the fire scene.
e Using a chemical that interferes with the chain reaction process of combustion;
Separation of fire components (inhibiting)
- By means of dry chemical.
Health hazard
The Hazards of LNG and Relevant Gasestoxic hazards to which personnel are exposed in tanker operations arise almost entirely from contact with gases of various kinds.
Skin contact
Many petroleum products, especially the more volatile ones, cause skin irritation and remove essential oils from the skin, leading to dermatitis. They are also irritating to the eye. Certain heavier oils can cause serious skin disorders on repeated and prolonged contact.
Ingestion
The risk of swallowing significant quantities of liquid petroleum during normal tanker and terminal operations is very slight. Petroleum has low oral toxicity to man, but when swallow it causes acute discomfort and nausea. There is then a possibility that liquid petroleum may be drawn into lungs during vomiting and this can have serious consequences, especially with higher volatility products such as gasoline and kerosene’s.
Inhalation
The main effect of petroleum gas on personnel is to produce narcosis. The symptoms include headache and eye irritation, with diminished responsibility and dizziness similar to drunkenness. At high concentration these leads to paralysis, insensibility and death.
Oxygen dificiency
The oxygen content of the atmosphere in enclosed spaces may be low for several reasons. The most obvious one is if the space is in an inert condition, so that carbon dioxide or nitrogen has displaced the oxygen. Also, chemical reactions such as rusting or the hardening of paints or coating can remove oxygen.
As the amount of available oxygen decreases below the normal 21 % by volume breathing tends to become faster and deeper. Symptoms indicating that an atmosphere is deficient in oxygen may give inadequate notice of danger. Most persons would fail to recognize the danger until they were too weak to be able to escape without help. This is especially so when escape involves the exertion of climbing.
Inert gas
Toxic Constituents
The main hazard associated with inert gas is its low oxygen content. However, inert gas produced by combustion either in a steam – raising boiler or in a separate inert gas generator contains trace amounts of various toxic gases, which may increase the hazard to personnel exposed to it.
The precautions necessary to protect personnel against this toxic hazard. These precautions do not include requirements for direct measurement of the concentration of trace constituents of flue gas, because gas freeing the atmosphere of a cargo tank from a hydrocarbon gas concentration of about 2 % by volume to 1 % LFL, and until a steady 21 % by volume oxygen reading is obtained, is sufficient to dilute these constituents to below their TLVs.
Nitrogen Oxides
Fresh flue gasses typically contain about 2 000 PPM by volume of mixed nitrogen oxides. The majority is nitric oxide which is not removed by water scrubbing. Nitric oxide reacts slowly with oxygen forming nitrogen dioxide. As the gas stands in tanks the total concentration of nitrogen oxides falls over a period of 1-2 days to a level of 10-20 PPM by solution of the more soluble nitrogen dioxide in free water, or by condensation, to give nitrous and nitric acids. Further decrease below this level is very slow. Nitric oxide is a colourless gas with smell at its TLV of 25 PPM. Nitrogen dioxide is even more toxic with a TLV of 3 PPM.
Sulfur Dioxide
Flue gas produced by the combustion of high sulfur content. Fuel oils typically contains about 2 000 PPM of sulfur dioxide (SO2). Inert gas system water scrubbers remove this gas with an efficiency, which depends upon the design and operation of the scrubber, giving inert gas with sulfur dioxide content usually between and 2 and 50 PPM.
Sulfur dioxide produces irritation of the eyes, nose and throat and may also cause breathing difficulties in sensitive people. It has a distinctive smell at its TLV of 2 PPM.
Carbon Monoxide
Carbon Monoxide is normally present in flue gas at level of only few parts per million, but abnormal combustion conditions and slow running can give rise to levels in excess of 200 PPM. Carbon monoxide is an odorless gas with a TLV of 50 PPM. It is insidious in its attack, which is to restrict oxygen uptake by the blood, causing a chemically induced form of asphyxiation.
Harmful properties of cargoes
Benzene and Other Aromatic Hydrocarbons
The aromatic hydrocarbons include benzene, toluene and xylene. They are components in varying amounts in many typical petroleum cargoes such as gasoline blending components, napthas and special boiling point solvents.
Hydrogen Sulfide
Many crude’s come out of the well with high levels of hydrogen sulfide, but this level is usual reduced by a stabilization may be temporarily reduced at times. Thus a tanker may receive a cargo of particular crude with a hydrogen sulfide content higher than usual. In addition some crude are never stabilized and always contain a high hydrogen sulfide can also be encountered other cargoes such as naphtha, bitumen and gas oils.
The TLV of hydrogen sulfide is 10 PPM.
| Table 1. Effects of the gas at concentrations in air excess of the TLV area | |
|---|---|
| 50-100 PPM | Eye and respiratory tract irritation after exposure of one hour |
| 200-200 PPM | Marked eye and respiratory tract irritation after exposure of one hour |
| 700-900 PPM | Rapid unconsciousness, death occurring a few minutes later |
| 1 000-2 000 PPM | Instantaneous collapse of breathing |
Gasoline Containing Tetraethyl Lead or Tetraethyl Lead
The amounts of tetraethyl lead (TEL) or Tetraethyl lead (TML) normally added to gasoline are insufficient to render the gases from these products significantly more toxic than those from unleaded gasoline. The effects of the gases from leaded gasoline are therefore similar to those described for petroleum gases in section.
First aid do’s and don’t’s
| Table 2. Effects of the gases from leaded gasoline | ||
|---|---|---|
| Asphyxiant | Symptoms | Treatment |
| LNG | Increased rate depth of respiration | Remove from exposure |
| LPG | Blueness of the skin (Cyanosis) | Apply critical respiration if required |
| Methane | Stertorous breathing – with a snoring sound | Apply external cardiac massage |
| Ethane | Loss of consciousness center | Loosen clothing |
| Propane | Give non-alcoholic drinks if desired | |
| Butane | Give non-alcoholic drinks if desired | |
| Nitrogen | Keep at rest | |
| Flue gas | Unless symptoms minor seek medical advice | |
| Table 3. Hazardous effect involve cargo handling risk concerning inerting and gas freeing | |||
|---|---|---|---|
| Crude Oil & Product | Liquefied Gas | Chemical | |
| Irritants | × | × | × |
| Narcosis | × | × | × |
| Asphyxia | × | × | × |
| Cold Burn | – | × | – |
| Chemical Burn | × | × | × |
| Toxic Systematic | × | × | × |
Toxicity
The ability of a substance to cause damage to living tissue, impairment of the central nervous system, severe illness or in extreme cases death, when ingested, inhaled or absorbed by the skin.
The amount required to produce these results vary widely with the nature of the substance and the time of exposure to it.
Acute toxicity
Refers to exposure to toxic substance for a period of short time.
Acute effect of toxicity
- Cause sudden death.
- Permanent injury.
Chronic toxicity
Exposure to toxic substance for a long duration (repeated/prolonged).
Chronic effect of toxicity
- Temporary immobility of the casualty.
- Symptoms of the effects can remain in human body.
- Can cause neurosis.
- Can aggravate into serious condition when complicate with other illness.
Systematic poisons and irritants
Toxins, carcinogens, hallucinogens, narcotic agents, can enter the body through abrasion, Skin Absorption, food intake.
Effects to the casualty – Toxins may interrupt organ functions and interference with the systems functions.
Effects on the senses
- Sight.
- Hearing.
- Taste.
- Smell.
- Immunity of the senses to stimuli.
- Cause nausea.
Common irritants
- Alkaline – skin irritants.
- BASE – eats human flesh.
- ACID – burn flesh.
Threshold limit value (TLV)
The maximum concentration of gases vapours, mist or sprays to which it is believed that nearly all persons on board maybe repeated by exposed.
TLV – TWA
Time weighted average concentration for an 8-hour/day or 40-hr/wk throughout working life.
TLV STEL
Short term exposure limit in terms of the maximums concentration allowable for a period of up to 15 minutes duration provided there are no more than 4 such excursion per day and at least 60 min between excursions.
TLV-C
The ceiling concentration which should not be exceed even instantaneously.
| Table 4. Examples | |
|---|---|
| CHEMICALS | TLV |
| Benzene | 10 PPM |
| Sulfuric Acid | 1 MG/M3 |
| Caustic Soda | 2 MG/M3 |
| Chlorine | 0,5 PPM |
| Hydrogen Sulfide | 10 PPM |
| Gasoline | 300 PPM |
Hazard to marine environment
Oil affects the marine environment in different ways. It blankets the surface, interfering with the oxygen exchange between the sea and the atmosphere; its heavier constituents blanket the sea floor, interfering with the growth of marine life; many constituent elements are toxic and get into the food chain; and oil on the beach interferes with recreation uses of that beach. Furthermore, oil may enter seawater-distilling inlets and it may be deposited on tidal mud flats, again with detrimental results.
| Effect of oil Pollution | It blankets the sea surface the see and, |
| Interfacing with the oxygen exchange between the see and the atmosphere. | |
| It heavier constituents blankets the sea floor. | |
| Chemical | Interfering with the growth of Marine life. |
| Toxic elements can get into the food chain. | |
| It interferes with recreation of the beach other amenities. |
Reactivity hazards
Special consideration have to be given to the possibility of chemicals undergoing a chemical or physical reaction during cargo handling and transport conditions thereby creating hazard.
Chemical reaction may produce heat which in turn may accelerate the reaction, may cause the release to a large volume of vapour and or/pressure rise, or may cause the formation of flammable and/or harmful vapours that otherwise would not be expected. In principal, the danger arising form chemical reactions are those of increased fire and health hazard.
Three main types of reaction have to be considered:
a Self-reaction and reaction with air where only the particular chemical itself is involved. Small amounts of other chemicals contact with certain metals may promote reaction. Polymerization is a common type of self-reaction.
b Reaction as a result mixing one chemical with another Neutralization of an acid with an alkali is a typical example of on chemical reacting with another.
c Reaction as a result of mixing with water. A cargo, which is self-reactive as in (a) or reacts with others in (b), may also react with water.
Self-reaction and reaction with air
Self-reaction or reaction with air can occur in the liquid, in the vapour or in both Reactions may be promoted by heat and by the presence of certain metals and other cargoes in small amounts. Control of temperature and the avoidance of unsuitable materials in the cargo system and contamination by even small amounts of other cargoes all contribute to the safe carriage of self-reactive cargoes.
Reaction in the liquid can be retarded by inerting or by adding to it a small amount of a specific chemical known as an inhibitor. Those cargoes, which in pure form can undergo a vigorous self-reaction (usually resulting in polymerization), may only be offered for transportation provided they contain an inhibitor.
An inhibitor added to the liquid generally will not retard reaction in the vapour or condensed vapour in the ullage space. Displacing air (oxygen) from the ullage space with inert gas will retard reaction of the vapour therein and inerting may be an additional requirement, even if the cargo contains an inhibitor.
General precautions for self-reactive cargoes and cargoes which react dangerously with air
a The cargo tanks and cargo handling system should be free of the metal components, which are listed in the date sheet as unsuitable.
b If the maximum cargo temperature during loading and on voyage needs to be controlled the Master should verify what the limit is and what means are available to ensure that it will not be exceeded during cargo handling and on the voyage. Cargo temperature should be measured regularly and cooling system put not operation when necessary. Loading should be stopped if the temperature of the cargo being received exceeds the limit.
c Even if temperature control is not a specific requirement it is recommended that self-reactive chemicals be never stowed in tanks directly adjacent to heated cargoes nor handled through pipelines, which pass through tanks containing heated cargoes.
d Also it is recommended that cargo tank tops are kept cool by water spray when an ambient temperatures are high, thereby to retard reaction in the vapour space.
e Before loading, the cargo tanks and cargo handling system should be thoroughly cleaned to remove other cargoes that may promote self-reaction of the cargo to be loaded.
Reactivity with other cargoes
To establish whether or not two cargoes will react dangerously together, the data sheets for both cargoes and cargo compatibility chart should be consulted.
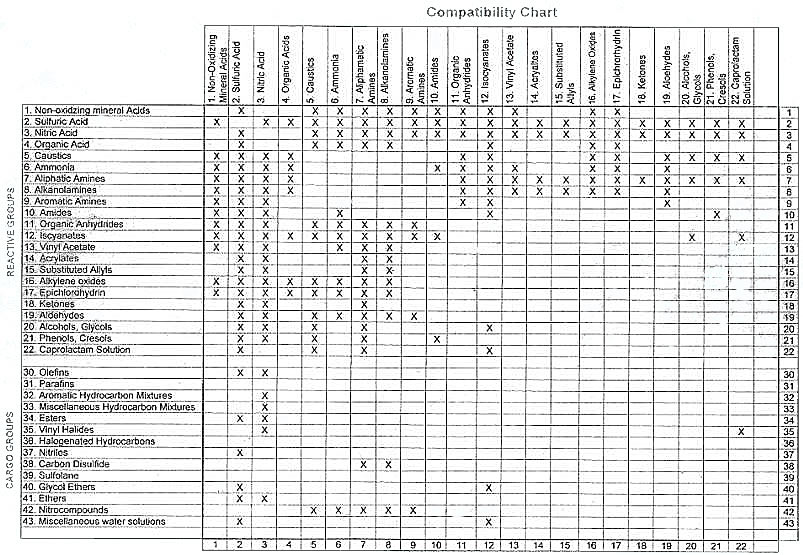
When the data sheet indicates that a dangerous reaction may result by mixing the cargo in question with another, «double» separation should be provided as greater security against accidental mixing.
«Double» Separation involves:
- A pumproom, Cofferdam – Definition and Pronunciationcofferdam or similar void space (which may be an empty cargo compartment) between tanks or compartments containing incompatible cargoes.
- At least one compartment loaded with a cargo containing with each, and separating the incompatible cargoes.
- Independent cargo pipelines to each compartment containing incompatible chemicals and which do not pass through any compartment containing other incompatible cargoes unless the pipelines is in a tunnel or similar arrangement.
- Independent vent systems on each compartment containing incompatible cargoes.
Reaction with water
The data sheet indicates if a dangerous reaction is possible between a chemical and water. If such a reaction is possible «double» separation between the chemical and water is recommended.
«Double» separation involves:
- Double skin as provided by a double bottom and side cofferdam between the cargo and the sea.
- A cofferdam or similar void space (which may be an empty cargo compartment) to separate the tank containing the cargo from tanks containing water.
- At least on compartment loaded with a cargo which is compatible with the cargo in question and with water.
- Pipelines serving the cargo tank should be independent from lines serving any tank containing water and should not pass through any tank containing water, or vice versa unless the pipeline is in a tunnel or similar arrangement.
- Vents systems serving the tanks containing the cargo should be independent from vent system serving tanks containing water.
Tank cleaning with water
If a Gas Freeing of Cargo Tanks on Liquefied Natural Gas Carrierscargo tank has contained a chemical, which reacts dangerously with water, tank cleaning with water should only be undertaken in the manner specified by the shipper or by some safe means. If toxic vapours are evolved on contact with water, then breathing apparatus may be required by those involved in the operation.
Preventing reactivity
Segregation of cargo:
If two or more cargoes are carried simultaneously, they are normally segregated from each other to avoid contamination and, in some cases, chemical reaction. If segregation is needed to avoid contamination shipper’s requirements on the degree if segregation are necessary and should be observed. If the same piping system is to be used for different cargoes, great care should be taken to ensure drainage and purging, if necessary between cargoes.
Separate reliquefaction system has to be used for different cargoes if possible. However, if there is a danger of chemical reaction, it is necessary to use completely segregated systems at all times. In such cases, regulations require “positive segregation” i. e. by means of removal spool pieces or pipe sections. If in doubt whether two cargoes are reactive, the data sheet for each cargo should be consulted and advice sought from shippers or other authority; if this advice seems inconclusive, the cargoes should be treated as incompatible and requiring «positive segregation».
Corrosion hazard
Corrosive liquids in general have three characteristics that require special consideration.
Corrosivity
Generally, they corrode normal construction materials at an excessive rate and need special materials for the cargo tanks and handling system to ensure safe containment.
Fire
When they corrode metals, hydrogen may be produced which forms flammable mixture with air. Contact with fibrous materials such as cloth sawdust, etc., may in some cases cause ignition of the material. Some corrosive liquids are combustible.
Health
They destroy human tissue causing serious damage, which may be permanent.
The characteristics vary in degree for different corrosive liquids and the data sheets should be referred to for particular guidance. Strict observation of the same overall precautions will ensure consistent safety in handling.
Corrosion Precautions
A corrosive liquid should not be loaded unless in can be verified that materials are suitable in the intended cargo tanks and associated handling system and that the liquid cannot come in contact with other spaces or systems where materials are not suitable.
The date sheet gives guidance on material which are considered suitable or unsuitable but expert advice should always be sought because the suitability of a material depends not only on the corrosive liquid but also on its concentration, temperature and often, on impurities that it may contain. Some acids become more corrosive as their concentration is reduced.
If the cargo requires heating it should be ascertained that the material on the heating coils might lead to serious corrosion at the temperature of the heating medium.
Read also: Properties of liquefied gases
Internal coils should be tested before loading starts as leakage of corrosive liquid into the coils may lead to serious corrosion within the heating system or machinery space equipment. Pressure in internal coils should be maintained at the level in excess of that of the cargo.
The cargo handling system should be such that, in the event of damage or wrong manipulation of valves, corrosive liquid cannot enter a space or other system where the constructional material will be corroded.
All gaskets jointing in the cargo handling system should be resistant to corrosive liquids and should always be kept tight, especially on decklines and pumprooms. Preferably, pump glands, flanges, fittings and valve stems should be provided with splash shields.
One of the greatest dangers to personnel is the unsuspected spray or leak of corrosive liquid.
Fire Precautions
Corrosive liquids should be regarded always as presenting a potential fire hazard because of the danger from hydrogen that may be produced when the liquid is in contact with metals.
All normal fire precautions should be taken and it is especially important that:
- Smoking is prohibited anywhere in way of the cargo space.
- Unauthorized work and hot work is prohibited in way of the cargo space.
- Pump room and spaces, if any, around the cargo tanks are kept properly vented.
- Strict precautions are taken when opening up spaces, if it is suspected that water has leaked into the corrosive liquid or alternatively corrosive liquid has leaked into any other space.
Because of the danger of ignition, materials such as cotton waste, sawdust, wood shavings, etc. should not be used for mopping up spillage of corrosive liquids.
Spillage Precautions
During cargo operations, water hoses should be connected and a water supply ready for immediate use and any spillage or leakage of corrosive liquid should immediately be washed away with a very large amount of water.
If for any reason a spillage has to be temporarily confined, sand or other inert materials should be used. Fibrous materials such as clothe, or wood should never be used.
Corrosive liquids should not be allowed to leak and collect in pumproom bilges. Any leakage should be washed out immediately with water.
Tank Cleaning Precautions
Tank, pipelines, pumps and all associated equipment should be drained and washed thoroughly with a large volume of water. A part from providing safe working conditions, complete removal of corrosive liquid is essential as any residue may cause serious corrosive accompanied by the formation of hydrogen.
Danger to personnel
- Some liquid cargoes are so corrosive that in contact with the skin will completely or partly destroy living tissue.
- Less corrosive liquids may only be irritating to the skin but can result in serious damage to the eyes.
- Corrosive liquids also produced corrosive vapors, which is also dangerous in contact with the skin, eyes and mucous membranes.
- Inhaling corrosive vapors may cause respiratory irritating or living lung damage.
Effects on constructional materials
- Generally corrosive liquids corrode normal construction materials at an excessive rate and need special materials for cargo tanks and handling system to ensure safe containment.
- A corrosive liquid should be loaded unless it can be verified that materials are suitable in the intended cargo tanks and associated handling system.
- The cargo data sheets gives guidance in materials, which are considered suitable and unsuitable, but expert’s advice should always be sought.
- The corrosive effect can depend on the liquid concentration, temperature and impurities it may contain.
- All parts of the cargo handling system including gaskets or joints should be resistant to the corrosive garage handled.
Techniques and precautions to prevent corrosion
On chemical tankers, all structural materials and innings used in cargo tanks and associated piping, valves and pumps must be suitable for the cargoes carried. Most chemical tankers usually have both coated and stainless steel tanks.
| Table 5. Cargo Tank Material Suitability with the Cargo | |||
|---|---|---|---|
| Coated by | Suitable for | Unsuitable for | Comments |
| Epoxy | Alkalis, glycols seawater, animal vegetable oil | Aromatic e. g. Benzene, toluene ethanol, methanol | Some coating can pick up product traces |
| Phenolic resins | Strong solvent polyurethanes | Better resistance than epoxy, but costs more | |
| Zinc Silicate | Aromatic hydro-carbon solvents, benzene, toluene alcohols ketones | Acids, alkalis, seawater, most vegetable oils and animal fats | Moisture in tank can result in some halogenated compounds reacting with cargo to produce |
| Polyurethane | All cargoes suitable for epoxy coatings, some compatible with zinc | Has smooth finish which cleans more easily than epoxy. Water soluble cargoes should not be followed by water until coating has been dried | |
| Rubber Lining | Highly cossrosive substances, phosporic acid, hydro chloric acid | ||
| Stainless Steel | Sulphuric acid, Nitric acid, Phosphoric acid, Caustic Soda (up to a certain temp, only), wine | Different grades of steel are used. Corrosion does occur but can usually be monitored. Seawater is especially corrosive to care must be taken to tank washing. | |
Cargo piping, valves and pumps are normally made of stainless steel. Stainless steel is resistant to most chemical cargoes even with very strong acids and bases. The main types of coating are resistant to groups of chemicals and it has its limitations.

