Gas carriers carrying liquefied natural gas (LNG), liquefied petroleum gas (LPG) and other gases require strict safety measures due to the dangerous nature of their cargo, which you can read about in detail below.
- Principal Hazards and Emergency Procedures
- Flammability
- Jet fires
- Liquid (pool) fires
- Vapour cloud explosion
- BLEVE
- Vaporisation of spilled liquid
- Rapid phase transitions (RPT)
- Uncontrolled release of vapour
- Vapour exposure
- Asphyxia (suffocation)
- Medical treatment for asphyxia or the effects of toxic materials
- Giving oxygen to a casualty
- Frostbite
- Chemical burns
- Other hazards of liquefied gases
- Emergency Planning
- The emergency plan
- Ship emergency procedures
- Terminal emergency procedures
- Removal of Ship from Berth
- Ship to Ship Cargo Transfer
- Hazards with the Use of Hoses and Marine Loading Arms (MLAs)
By adhering to these safety practices, gas carriers can minimize risks and ensure the safe transport of hazardous cargoes.
Principal Hazards and Emergency Procedures
A hazard is created by the properties of a dangerous substance or the dynamics of a physical situation that have the potential for creating harm to human health and/or the environment.
One of the main hazards in the liquefied gas industry is the flammability of the majority of cargoes. One of the primary concerns, therefore, will often be to prevent the formation of a flammable mixture. This is addressed by preventing loss of containment, eliminating ignition sources and by maintaining an inert atmosphere. It is important to understand the technical design of a gas carrier and terminal, and the specific operational measures that are in place, to start to be in a position to create an effective response strategy.
A significant hazard is due to the pressure and low temperature at which liquefied gas may be carried. LPG may be carried at ambient temperature in pressure tanks, but LNG is mostly carried at cryogenic temperatures and atmospheric pressure, as it does not remain as a liquid at ambient temperatures.
Another potential hazard is the toxicity of some of the liquefied gas products. When liquefied gas is contained within tanks and pipelines it is safe and poses little risk, ie it is a stable cargo that does not polymerise or decompose to form an unstable substance. It is generally only in the event of a loss of containment that liquefied gas becomes a hazard. This is why gas carriers and terminals have design considerations to guard against loss of containment. Very small amounts of operational losses (ie drips and operational venting) are considered in the design by ensuring that the cargo area (areas where the presence of liquefied gas is expected and are so classed as Zone 1 under the IGC Code hazardous area classification) is free from sources of ignition and that there is the required splash protection.
The consequences from severe structural damage to a liquefied gas carrier may be more serious than for similar incidents involving other ship types. Every aspect of the operation of liquefied gas carriers in port areas, including the location of holding anchorages, the transit to and from sea, the location of the berth and management of other marine traffic while the ship is alongside the berth, requires careful analysis and detailed planning to help to eliminate any credible probability of the ship sustaining serious structural damage.
At the jetty, one of the main hazard sources is the cargo transfer piping, ie marine loading arms (MLAs) and associated piping at the jetty head. The jetty is a designated gas hazardous zone, which means sources of ignition will usually be strictly controlled.
When liquefied gases are safely contained in the tanks and piping of a liquefied gas carrier or terminal there is usually no immediate hazard. Loss of containment of liquefied gas, or fire impingement on a liquefied gas in a tank or pipeline, could lead to a hazardous situation.
For a fire to exist there should be oxygen, flammable vapour and a source of ignition – the three parts of a fire triangle. The design of gas carriers and terminals is intended to restrict the co-existence of two parts of the fire triangle at any given point in time. Where there is oxygen present, such as on the cargo deck area, flammable vapour and ignition sources are restricted by the design. Where there is flammable vapour present, such as in cargo tanks, oxygen and ignition sources are restricted by the design.
The design of gas carriers and terminals require elimination of ignition sources in areas where there is a risk of a leak. Therefore, a leak does not necessarily result in an ignition and the vapour needs to travel to the nearest ignition source for a fire or explosion to occur.
The design of gas carriers and jetties generally makes it difficult for vapour to collect in a confined area, so an explosion caused by the ignition of confined vapour is unlikely for most leaks. Compressor rooms and motor rooms can create the conditions for vapour to accumulate, so the IGC Code requires these spaces to have multiple safety barriers to prevent an incident.
To create a credible response strategy, all possible scenarios should be examined in a structured manner. For liquefied gas operations at a terminal, the following will usually be considered amongst other factors: unignited release of vapour, unignited release of liquid and cold spill.
These may lead to, for example, fires (jet or pool), explosions (vapour cloud) (see point “Vapour cloud explosion” below) and BLEVE (see point “BLEVE” below), over-pressure (see article Equipment and cargo system of LNG onshore terminals“Pressure relief venting”) and exposure injury (see points “Asphyxia (suffocation)” and “Other hazards of liquefied gases” below). Vapour clouds of LNG and LPG are non-toxic, although there is still the danger to personnel due to asphyxiation or a fire (unconfined).
A series of large-scale liquefied natural gas pool fire and cryogenic damage tests were conducted by Sandia Notional Laboratories, from 2008 through 2011, to expand LNG safety efforts and address various public safety risks posed by LNG carriers transiting to terminals. These studies generally concluded that:
- the risks from accidental spills are small and manageable;
- large non-ignited LNG vapour releases are unlikely;
- that cascading events are not expected to greatly increase hazard ranges.
The most significant safety related impacts are usually within a relatively short distance (approx 500 m) from the source of the spill.
Full details of the test programme and results are available from Sandia National Laboratories, including publications “The Phoenix Series Large Scale LNG Pool Fire Experiments” SAND2010-8676 (Reference 2.78), “Guidance on Risk Analysis and Safety Implications of a Large Liquefied Natural Gas (LNG) Spill Over Water” SAND2004-6258 (Reference 2.79) and “Recommendations on the Prediction of Thermal Hazard Distances from Large Liquefied Natural Gas Pool Fires on Water for Solid Flame Models” SAND2011-9415 (Reference 2.80).
Flammability
If a gas released to atmosphere is exposed to a source of ignition at the point it is within its flammable range, it will burn. Depending on the conditions under which combustion takes place, some degree of over-pressure will occur due to the rapid expansion of the heated gas.
A liquid spill or vapour cloud burning over open water will usually not develop over-pressure due to the unconfined nature of the surroundings. At the other extreme, the ignition of vapour within on enclosed space may rapidly create on over-pressure sufficient to burst the boundaries. Between these two extremes, ie in cases of partial confinement such as might occur in shore plant and equipment, ignition may produce over-pressures sufficient to cause substantial damage, escalating the hazard and its consequences.
A leakage of liquid or vapour from a pipeline under pressure will burn, if ignited, as a jet that will continue as long as fuel is supplied.
Jet fires
Small leaks from pump glands, pipe flanges or from vent risers will initially produce vapour. This vapour will not ignite spontaneously but, if the escape is large, there may be a risk of the vapour cloud spreading to a source of ignition. Should a gas cloud occur, ignition will usually be prevented by closing all openings to hazardous areas and by the vapour cloud being directed or dispersed away from ignition sources by means of fixed or mobile water sprays (see article “Water” below). If ignition does occur, it will almost certainly flash back to the leak. Leaks from pipelines are likely to be under pressure and, if ignited, will give rise to a jet flame. Emergency shutdown of pumping systems and closure of ESD valves will usually already have occurred, but pressure may persist in a closed pipeline until the liquid trapped within has been expelled through the leak. In such a case the best course of action may be to allow the fire to burn out. The alternative of extinguishing the fire has a high risk of further vapour cloud production and flash-back causing re-ignition. While the fire is being allowed to burn itself out, the surroundings will usually be protected with cooling water.
Liquid (pool) fires
Given the lower density of liquefied gases, any spill of liquefied gases to water will result in the liquefied gas floating on the water surface. Other than ammonia, liquefied gas hydrocarbon constituents are not soluble in water and, therefore, no water contamination occurs. Spills on water will be unconfined under most release conditions, ie the liquid pool will continue to spread until the vaporisation rate equals the release rate or until ignition occurs and the burning rate equals the release rate.

Significant pool fires are generally considered not likely on ships’ decks because the amount of liquid that can be spilled in such a location is limited. The arrangement of the ship’s deck, with its camber and open scuppers, will allow liquid spillage to flow quickly and freely away over the ship’s side. Prompt initiation of ESD procedures should help to limit the spillage of liquid cargo.
A water curtain is fitted on LNG ships to provide a warming flow down the side adjacent to the cargo manifold. This is to limit the possibility of brittle fractures that would be the result of a spill of the cryogenic liquid cargo.
A liquid spillage on shore, from tank or pipeline ruptures, may involve large quantities but should be contained in bunded areas or culverts. Any ignition of the ensuing vapour cloud would then result in a pool fire. The flame height from such a fire, in the absence of wind, is as illustrated in Figure 2, which also illustrates the effect of wind in deflecting the axis of the flame and in shortening flame-length.
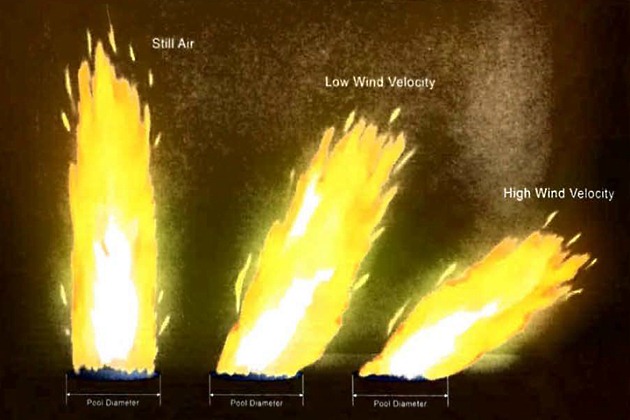
The emissive power of a flame surface increases with pool diameter. LNG vapours burn, in the initial stages, with a comparatively clear flame. LPG, however, burns with a greater production of soot and, as a result, maximum surface emissive powers are lower than for LNG. Heat radiation levels from both LNG and LPG pool fires dictate that unprotected personnel will usually need to escape from the immediate vicinity as quickly as possible.
Read also: Essential Steps for Preparing LNG Tanks for Cargo Loading on LNG Vessels
Heat radiation from a fire decreases rapidly as the distance from the fire increases. Pool fire hazards are localised and, as a result, thermal radiation effects (burns) are typically confined to within one or two pool diameters from the edge of the flame. Thermal radiation is absorbed by any water moisture and carbon dioxide present in the air. The human body will feel extreme pain on bare skin after only 10 seconds of incident radiation of 6 kW/m2 and will suffer severe blistering after 10 seconds exposure to 10 kW/m2. Incident radiation greater than 10 kW/m2 will quickly vaporise polyvinyl chloride (PVC) cables and will seriously affect fibreglass lifeboats.
The estimation of safe distances from a pool fire involves complex factors but, for a large pool fire, such safe distances are likely to be some tens of metres. Because of the damage that radiation can inflict on surrounding tanks and plant, such equipment is always protected (often by insulation or by remotely operated water deluge systems). Also, the bunds and culverts where pool fires may occur are often provided with remotely operated fire-fighting equipment, such as a high expansion foam system that can rapidly build up and maintain a depth of foam to control the rate of burning.
Typical locations where a pool of liquefied gas can form are at the ship’s manifold drip tray, in the sea or in a containment area at the terminal.
Vapour cloud explosion
The gases produced by combustion are heated by the reaction. In open spaces, gas expansion is unrestricted and combustion may proceed without undue over-pressures developing. If the expansion of the hot gases is restricted in any way, pressures will rise and the speed of flame travel will increase. This depends upon the degree of confinement encountered. Increased flame speed gives rise to a more rapid increase in pressure, with the result that damaging over-pressures may be produced. Even in the open, if the confinement resulting from surrounding pipework, plant and buildings is sufficient, the combustion can take on the nature of an explosion. In severely confined conditions, such as within a building or ship’s tank where the expanding gases cannot escape, the internal pressure and its rate of increase may be sufficient to burst the containment. Here, the explosion is not due to high combustion rates and flame speed, it is more a result of the surge of high pressure as the containment ruptures (see article “Properties of liquefied gasesFlammability/flammable range” for further discussion).
BLEVE
A boiling liquid expanding vapour explosion (BLEVE) is a phenomenon associated with the sudden and catastrophic failure of a pressurised vessel. The container may, for example, fail for any of the following reasons:
- mechanical damage;
- corrosion;
- excessive internal pressure;
- flame impingement or metallurgical failure.
In the most common example of a BLEVE, a fire increases the internal tank pressure and flame impingement reduces the mechanical strength of the tank, particularly at the part of the vessel not cooled by internal liquid. The tank suddenly, and catastrophically, fails and pieces of the shell can be propelled considerable distances. On rupture, the sudden decompression produces a blast and the pressure immediately drops. At this time the liquid temperature is well above its atmospheric boiling point and, accordingly, it spontaneously boils off, creating large quantities of vapour that are thrown upwards along with liquid droplets. Where the gas/air mixture is within its flammable limits, it will ignite from the rending metal or the surrounding fire to create a fireball. The sudden release of gas provides further fuel for the rising fireball and the rapidly expanding vapour produces a further blast and intense heat radiation.
BLEVE incidents have occurred with rail tank cars and road vehicles and there have been a number of terminal incidents. As at the date of publishing, there have been no instances on liquefied gas carriers. Under the IGC Code, pressure relief valves (PRVs) sized to cope with surrounding fire (as for shore tanks), the allowance for increased fill limits in Type C tanks (see article “Preparation of loading and unloading operations for LNG/LPG carriersCargo tank loading limits“) and the fitting of fixed water spray systems as for shore tanks, may help to limit the risk of BLEVE incidents. While the sizing of the PRVs does not specifically prevent BLEVE, it does prevent over-pressurisation of the tank. If the PRVs are working as designed, however, during the course of a fire the inventory from the tank is discharged such that the liquid level drops and no longer adequately cools the upper parts of the tank.
The chance of a fire occurring in the enclosed space beneath a pressurised ship’s tank is much smaller than on an equivalent tank situated on shore, which minimises the possibility of a surrounding fire occurring on a ship and the possibility of a BLEVE occurring on a gas carrier.
Vaporisation of spilled liquid
In the unlikely event of cargo escape from fully-refrigerated containment, the liquid is already cold so it is close to or at atmospheric pressure. On escaping it contacts the sea, the ship’s deck or the ground, which will be at ambient temperature. The temperature difference between the cold liquid and the material it contacts provides an immediate heat transfer into the liquid that will, in turn, create a rapid generation of vapour.
If the spill collects in a pool, heat will be absorbed from the container (eg a drip tray or the ground) and this will reduce the temperature difference for some time. Eventually, these temperature differences will stabilise and the evaporation will continue at a reduced rate. The liquid will continue to boil-off until it has evaporated completely. It is quite possible that during the process the liquid temperature will “sub-cool“, ie fall below the atmospheric pressure saturation temperature.
In the case of cargo spillage onto the sea, the strong convection currents in the water will maintain the initial temperature difference and evaporation is likely to continue at a high rate. The large quantities of relatively cold vapour produced will cause condensation of the moisture from the air, forming a visible cloud of water vapour that looks white in colour, like mist or fog.
Spillage from pressurised containment is different. On escape the liquid may be close to ambient temperature, but the higher pressure prevailing in the containment space drops rapidly to atmospheric. This rapid pressure drop causes very high rates of evaporation, with the heat required mainly coming from the liquid itself. This process is called “flash evaporation” and a large proportion of the liquid may flash off (or evaporate) in this way. As a result, any liquid remaining cools rapidly to, or lower than, its saturation temperature at atmospheric pressure.
If cargo escapes under high pressure (eg from a pump discharge gasket failure), much of the liquid will spray into the atmosphere as small droplets, which will in turn take heat from the air and condense the water vapour in the atmosphere to form a white visible cloud. The liquid droplets will soon vaporise, which causes further cooling and will continue the white cloud formation for longer. If any pools of liquid are formed, these will attain an equilibrium temperature and evaporate until no more liquid remains.
If the cargo that leaks is flammable, the main hazard caused by the escape is the generation of large vapour volumes that create a flammable cloud when mixed with air. The white vapour cloud can give some indication of the flammable zone, but it may not coincide exactly. Similar considerations apply to any toxic cargo leaks.
In addition to the hazards of vapour/air mixtures, the cold liquid can cause frostbite on human tissue and may cause metals that come into contact with it to become brittle.
Rapid phase transitions (RPT)
During liquid spills of LNG on water, a phenomenon that is observed early on during liquid pool development is a rapid phase transition (RPT). An RPT is the very rapid (near spontaneous) formation of vapours as the cold LNG is vaporised from heat gained from the underlying spill surface.
When a liquid at its saturation temperature is heated slowly, the phase change from liquid to vapour will occur at a steady rate. If the heating is very rapid, owing to a large temperature difference between heat source and liquid, it is possible for the liquid to become meta-stable as a superheated liquid up to a stability limit. For pure methane, the atmospheric boiling point is minus 161,5 °C (-161,5 °C) and the thermal stability limit is minus 101,7 °C (-101,7 °C). When the limit is reached, the meta-stable condition can no longer be maintained and on RPT to vapour occurs. The excess energy stored in the liquid is released instantaneously and localised over-pressurisation is created. It is described as o “physical explosion“, or a “flameless explosion“, because no combustion takes place. The energy release rate is significantly less than if stoichiometric combustion occurs, although RPTs can still be potentially damaging.
The prediction of RPTs is not precisely understood but the following factors may influence the likelihood:
- “Richer” LNG mixtures are more prone to RPT then lean ones, (noting that, in the event of a spill, the methane will boil-off preferentially from the LNG so the mixture becomes more rich as the event proceeds with time);
- physical agitation of the LNG/water interface promotes RPT activity (ie jetting or a high rate spillage of LNG into water).
RPTs can range from small “pops” to big bangs. For instance, spillage into a drip tray is considered unlikely to lead to a large event simply because the capacity of the heat source (water in the drip tray) is limited. Nevertheless, small “pops” can project LNG into the air, over the edge of the drip tray, to splash onto the adjacent unprotected steel structure. It also increases the hazard to any personnel unfortunate enough to be nearby. For this reason, manifold drip trays will usually be kept as dry as reasonably practical during all liquefied gas cargo transfers except for ammonia cargoes, even though there is no risk of RPT with LPG cargoes. For ammonia cargoes, a small amount of water in the manifold drip tray will rapidly absorb any small drips.
Uncontrolled release of vapour
The vapour resulting from an uncontrolled release from tank vent masts will, in the case of LPG, be denser than air and will be carried downwind before being diluted to below its flammable or toxic limits. On an LNG carrier the vapour will initially be cold and denser than air and the vapour will travel downwind before warming, becoming lighter than air and dispersing. If the vapour cloud reaches a source of ignition, which may include a lightning strike, before it is diluted below its flammable limits it will burn back to the source and cause a fire at the head of the vent mast. For LPG the burn back may result in explosive over-pressures, particularly in confined spaces.
Vapour exposure
Toxicity
Toxicity is the degree to which a substance may cause harm to living organisms. Illness or, in extreme cases, death may occur following exposure to harmful gases or liquids. The IMDG Code states that “The word “toxic” has the same meaning as “poisonous“”.
| Table 1. Toxicity classifications | |||
|---|---|---|---|
| USA EPA Toxicity Classes | EU Toxicity Classes | World Health Organisation Toxicity Classes | IMO IMDG Code |
| Class I: most toxic | Class I: very toxic | Class 1 – a: extremely hazardous | Class 6.1 – Toxic substances These are substances liable either to cause death or serious injury or to harm human health if swallowed or inhaled, or by skin contact. |
| Class II: moderately toxic | Class II: toxic | Class 1 – b: highly hazardous | |
| Class Ill: slightly toxic | Class Ill : harmful | Class 2: moderately hazardous | |
| Class IV: practically nan-toxic | Class IV: corrosive | Class 3: slightly hazardous | |
| Class V: irritant | |||
| Class VI: sensitising | |||
| Class VII: carcinogenic | |||
| Class VIII: mutagenic | |||
Occupational exposure limit (OEL)
An occupational exposure limit is o recommended maximum concentration level to which a worker should be exposed. OELs are usually established by international, national or local regulation, with values usually quoted in ports per million (ppm) of vapour in air by volume or as milligrams of substance per cubic metre of air (mg/m3). The most widely quoted OEL system is that of the American Conference of Governmental Industrial Hygienists (ACGIH), which uses the term threshold limit value (TLV ©), but OEL systems from advisory bodies in other countries are generally similar in structure.
There are three categories of OEL:
- TWA. This is the time weighted overage and is the most commonly quoted value. It is the overage concentration of vapour in air that may be experienced for on 8 hour day or 40 hour week, throughout a person’s working life, without adverse effects occurring. In the UK this is also referred to as the workplace exposure limit (WEL).
- STEL. This is the short term exposure limit and is the maximum safe concentration of vapour in air for exposures of up to 15 minutes, provided there are no more than 4 exposures per day with at least 1 hour between each.
- STEL-C. This is known as the ceiling concentration of the vapour in air and its value should never be exceeded. Generally, it is substances that are fast acting, such as ammonia and chlorine, that are given a STEL-C. It is always good operating practice to keep all vapour concentrations to on absolute minimum to limit personal exposure.
While serious health effects may not usually be likely as a result of exposure to OEL concentrations, OELs should not be generally regarded as “safe” limits. It is industry practice to keep all vapour concentrations to an absolute minimum and to reduce exposures to as low as reasonably practicable (ALARP).
Effects of exposure to toxic gases
Many gases can act as poisons when exposure occurs through inhalation, skin contact or ingestion. Symptoms of acute exposure may include irritation of skin/eyes/throat, breathing difficulties, disorientation, dizziness, vomiting, chemical burns, headaches, laboured movements and unconsciousness.
Long term effects may occur. For example, vinyl chloride has carcinogenic effects.
Tables 2 and 3 give examples of the harmful properties of some common gases and their vapours, as per the ILO International Chemical Safety Card (ICSC database).
| Table 2. Main liquefied gases, including their flammable and toxic hazards | |||||||
|---|---|---|---|---|---|---|---|
| Cargo Vapour in Air | Toxic Effects of Vapour or Liquid | ||||||
| Substance | Flammable | Toxic | ICSC OEL-TWA (ppm) | Corrosive/Irritant | Effect on Nervous System | ||
| Hydrocarbon Liquefied Gases | ↕ | Methane | Yes | No | (*) | No | No |
| Ethane | Yes | No | (*) | No | Yes | ||
| Propane | Yes | No | 1 000 | No | Yes | ||
| Butane | Yes | No | 1 000 | No | Yes | ||
| Ethylene | Yes | No | 200 | No | Yes | ||
| Propylene | Yes | No | (*) | No | Yes | ||
| Butylene | Yes | No | Not established | No | Yes | ||
| lsoprene | Yes | No | Not established | No | Yes | ||
| Chemical Gases | ↕ | Butudiene | Yes | Yes | 2 | Yes | Yes |
| Ammonia | Limited | Yes | 25 | Very | No | ||
| Vinyl chloride | Yes | Yes | 1 | Yes | Yes | ||
| Ethylene oxide | Yes | Yes | 1 | Very | Yes | ||
| Propylene oxide | Yes | Yes | 2 | Very | Yes | ||
| Chlorine | No | Yes | 0,5 | Very | Very | ||
| (*) = These gases do not hove established OELs. These gases, which are comparatively non-toxic in character, are known as simple asphyxiont gases and will kill when their concentration in air is sufficient to displace the oxygen needed to sustain life. | |||||||
The last two columns of Table 2 show how a liquefied gas may affect a person. Broadly, the initial toxic effects on the human body may be corrosive or narcotic (affects the nervous system). In certain cases both may apply. In the case of a corrosive compound, depending on exposure and toxicity, the effects may be minor or major. In the case of minor effects, only limited irritation of the skin, eyes or mucous membranes may be felt. An example of a more serious case may be that debilitating effects on the lungs are experienced. In the case of exposure to a narcotic gas, the major initial effect is on the body’s nervous system, with severe disorientation and mental confusion the result. The corrosive and narcotic effects are of help in identifying the gas to which a person has been exposed and, additionally, they help in identifying proper medical treatment.
| Tables 3. Health data – cargo inhibitors | |||||
|---|---|---|---|---|---|
| Cargo Inhibitors | Toxic Effects | ||||
| Substance | Flammable | Toxic | ICSC OEL-TWA | Irritant | Effects on Nervous System |
| Tetrahydroquinone (THQ) | Limited | Yes | 2 mg/m3 | Very | Yes |
| Tertiary butyl catechol (TBC) | Limited | Yes | Not established | Very | – |
Detailed information on toxicity characteristics, amongst others, of a chemical can be found in the chemical’s safety data sheet (SOS) (see article “Personal health and safety crew members on board a gas carrierSafety Data Sheets (SDS)“).
Table 3 provides similar information to that shown in Table 2, but covers the potential hazards of cargo inhibitors. Information on the types of inhibitor used for particular cargoes is given in article “Properties of liquefied gasesFormation of polymers or dimers“.
While not an inhibitor, ethyl mercaptan is used in the LPG trade as an odorant. As produced LPG has almost no odour, ethyl mercaptan is added and the resulting strong, unpleasant odour is readily detectable, serving as a warning in the event of an LPG leak. Ethyl mercaptan is both extremely flammable and toxic, and the SDS should be consulted when it is used.
The IGC Code (Chapter 19) provides a list of the more hazardous products and their special requirements, such as the toxic alarm or flammable alarm required for the carriage of certain cargoes.
Asphyxia (suffocation)
The human body requires air with an oxygen content of approximately 20,9 % by volume. When the oxygen concentration falls below about 19 % by volume mental confusion and impaired mobility rapidly occur. Mental confusion is particularly dangerous because the victim may not be capable of understanding the situation and they are likely to require help to escape from a hazardous location.
At lower oxygen levels, unconsciousness takes place rapidly and, if the victim is not removed quickly, permanent brain damage or death may result.
In most cases, oxygen deficiency occurs in enclosed spaces with the following conditions:
- when large quantities of cargo vapour are present;
- when large quantities of inert gas or nitrogen are present;
- where rusting of internal tank surfaces has taken place.
In addition to displacing oxygen, combustion-generated inert gas also has the added danger of potential exposure to carbon monoxide (see article “Properties of liquefied gasesThe chemical compatibility of cargoes with inert gas or nitrogen“).
Symptoms of asphyxia
The symptoms of asphyxia may include:
- breathing difficulties (this is the main symptom that may result in a blue appearance of the skin);
- increased rate and depth of respiration;
- strenuous breathing (with a snoring sound);
- loss of consciousness.
Nitrogen asphyxia
There are risks associated with nitrogen, including that of complacency as it is non-toxic and comprises the bulk of the air which we breathe. However, nitrogen is safe to breathe only when mixed with the appropriate amount of oxygen.
Nitrogen asphyxiation may occur in open spaces if it is inhaled in an area where inerting is taking place. All such spaces will normally be clearly labelled with warning signs stating the danger. The crew will usually be trained in the procedures for using nitrogen and to ensure they are familiar with the ship and areas where nitrogen may accumulate.
- a compressed air breathing apparatus should be used when o crew member could be exposed to nitrogen;
- areas where personnel may be exposed to nitrogen should be restricted only to the personnel who are directly involved in the operation. Personnel working in these areas should wear o personal oxygen meter;
- if a space may be oxygen-deficient, all tank openings (besides those used as part of the gas-freeing operation) should be kept closed until the space has been cleaned, ventilated, and tested to ensure it is gas-free. Openings to other spaces, that could be oxygen-deficient, such as o nitrogen generator room, should be kept closed and secured, and verified as safe prior to entering;
- oxygen resuscitation units should be readily available in case of hypoxia due to nitrogen inhalation;
- when nitrogen is provided from shore, the pre-transfer meeting between the vessel and shore personnel must include o discussion addressing the operational and safety hazards related to the operations to take place.
See the CDI Publication “Best Practice Recommendation Regarding the use of Nitrogen” (Reference 2.81) for further guidance.
Entry into oxygen deficient spaces
Entry to any enclosed space will usually be prohibited, without proper personal protective equipment (PPE), including self-contained breathing apparatus (SCBA), until an oxygen content of 20,9 % by volume is established. Oxygen levels can be determined by sampling the atmosphere from a number of points using an oxygen analyser. Sample points will normally be at different levels and widely dispersed within the space. Tests for hydrocarbon gas and carbon monoxide may also be required.
If tank entry is absolutely necessary and a gas-free condition cannot be ensured, personnel entering the space will need to be protected by conducting a risk assessment, following the Company’s enclosed space entry procedure, and completing on enclosed space entry permit and a permit to work (PTW) and using breathing apparatus.
For further discussion on entry into enclosed spaces, see article “Personal health and safety crew members on board a gas carrierEntry into Enclosed Spaces“.
Medical treatment for asphyxia or the effects of toxic materials
In cases where someone has not been completely overcome by the effects of exposure to gas or toxic materials, symptoms will be giddiness, confusion and an inability to stand up properly or walk straight.
In many cases, a person overcome by exposure to gas or toxic materials will have managed to leave the affected area in an attempt to summon help, only to collapse unconscious nearby.
Medical treatment for exposure to gas will depend on the amount inhaled, the period of exposure and the nature of the gas or toxic materials. However, treatment typically consists of:
- removing the casualty to a safe area;
- providing artificial respiration (airways, breathing, circulation (ABC));
- administering oxygen (see point “Vapour exposure” above).
Professional medical advice and treatment should always be obtained where a person has been overcome by gas.
Further advice on these issues is available in the SDS carried onboard (see article “Personal health and safety crew members on board a gas carrierSafety Data Sheets (SDS)“) and the “Medical First Aid Guide (MFAG)“, which is in the IMDG Code Supplement (Reference 1.15).
The advice given in MFAG deals with the chemicals and substances detailed in the IMDG Code and covers: diagnosis of poisoning, first aid, poisoning complications, toxicity hazards, emergency treatment, chemical tables including indices and a list of medicines.
Treatment for gas poisoning or asphyxiation
The main points to be aware of when treating casualties for gas poisoning or asphyxiation are:
- remove the casualty at once from the dangerous atmosphere – ensure that rescuers are equipped with SCBA (see article “Breathing apparatus”) so that they are not overcome as well;
- check the casualty is breathing by tilting the head backwards as far as it will go to relieve obstructions and listen for breathing with the rescuer’s ear over the casualty’s nose and mouth;
- if the patient is not breathing:
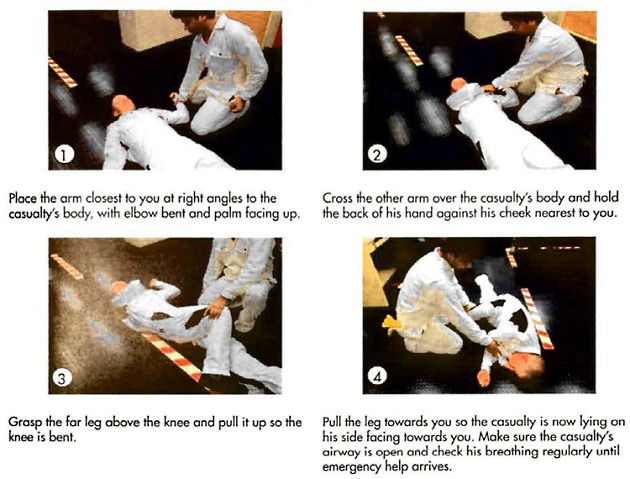
- give artificial respiration (ABC) immediately if the casualty is breathing but unconscious (casualty is unconscious with bluish skin), (Figure 4);
- place the casualty in the recovery position (Figure 4);
- check there are no obstructions in the casualty’s mouth (remove any false teeth);
- insert a mouth airway piece and leave in place until the casualty regains consciousness;
- administer oxygen (see point “Giving oxygen to a casualty” below);
- keep the casualty warm; give no food, drink or any morphine or stimulant, and do not allow the casualty to smoke.
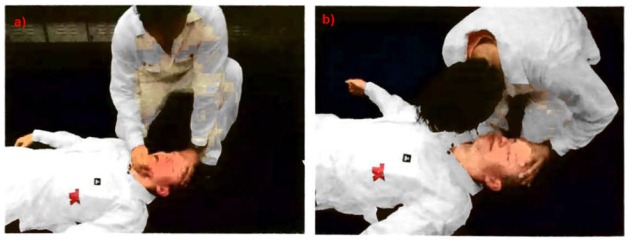
a – open the airway with a head tilt-chin lift manoeuvre; b – look, listen and feel for signs of breathing.
Where there are no signs of meaningful breathing – chest compressions will normally need to be started
If the casualty is conscious but having difficulty breathing:
- place the casualty in a high sitting-up position and keep warm;
- administer oxygen (see point “Giving oxygen to a casualty” below).
If breathing does not improve despite these measures, or the casualty’s condition deteriorates rapidly, obtain medical advice.
In cases of mild exposure the casualty should be allowed to rest until a full recovery has been made. If the casualty has to be Medivac’d from the vessel, the authorities will be given as comprehensive a report as possible detailing the type of gas and any other relevant information.
Giving oxygen to a casualty
Oxygen resuscitators are used to provide oxygen-enriched respiration to assist casualties in recovery in cases where they have been overcome by oxygen deficiency or toxic gas.

Modern automatic resuscitator sets are supplied with lightweight cylinders, making them lighter and easier to carry, so that they can be taken into enclosed spaces to give immediate treatment to a casualty. Automatic resuscitator sets can manage the casualty’s breathing, leaving those attending free to deal with other injuries or problems.
It will be interesting: Recommended methods for calculating LNG
Oxygen resuscitators comprise a face mask, pressurised oxygen cylinder and automatic controls to avoid injury to the casualty in the event of any obstructions to his airways. Pressure release valves and audible warning devices advise the operator if there is any airway obstruction.
All resuscitators will usually be inspected and tested at least weekly (or at shorter intervals as the manufacturers may recommend) and the inspection recorded in the medical log.
Care of oxygen resuscitator sets
- oxygen cylinders will usually be stowed in permanent storage racks in an upright position. These cylinders will be filled to capacity and ready for immediate use. All cylinders will normally be labelled with a warning tog indicating that they contain “MEDICAL OXYGEN“;
- empty cylinders will not usually be stowed in the some storage rocks as full cylinders;
- core needs to be exercised when handling oxygen to prevent contact with oil, grease, rubber, organic lubricants, or other materials of on organic nature;
- oxygen cylinders will usually be hydrostaticolly tested at least once every five years;
- never cover on oxygen cylinder with any material;
- never use oxygen fittings, valves, regulators, or gouges for any service other than for oxygen;
- defective oxygen equipment should always be repaired or replaced by authorised agents or manufacturers.
Cautions when using oxygen
When used in hazardous cargo areas, the risk of explosion or fire caused by an oxygen-enriched atmosphere can be reduced by changing the oxygen cylinder to a compressed air cylinder.
It is dangerous to connect/disconnect high pressure oxygen cylinders in an explosive atmosphere. Attention is drawn to the dangers of explosion caused by greasing or oiling any part of high pressure oxygen apparatus. Resuscitator sets must not be taken into a dangerous space unless fitted with a regulating valve. Smoking or any naked flame will not be allowed in the same room during the administration of oxygen because of the risk of fire.
The oxygen cylinders will usually be opened by turning both the valves fully anti-clockwise. The airway tube will normally be fitted into the throat and the facemask placed over the nose and mouth.
If oxygen can be heard escaping from the airway passage relief valve, or the warning whistle sounds, it is sensible to remove the face mask and check for obstructions, such as blood or vomit, in the casualty’s mouth or the face mask.
The casualty can now be placed on a stretcher and removed from the space. Resuscitation will continue until either the oxygen supply is exhausted or the casualty is able to breathe unaided.
Typically, resuscitator sets are fitted with two oxygen cylinders so that an empty cylinder can be replaced without interrupting operation of the set.
Frostbite
Frostbite or cold burns can occur when skin comes into contact with extreme cold that causes the tissues to freeze. Liquefied gases may be at a temperature that is low enough to cause frostbite, as well as irritation and chemical burns, as shown in Table 4.
Table 4. Health data – liquids сделать картинкой
Prevention of frostbite
Areas containing liquefied gases, or other extremely cold substances, should not be approached without appropriate PPE. Thermal protective clothing will usually always need to be worn if refrigerated or cryogenic liquids are to be handled. Face and eye protection, along with additional protection for hands and feet, may be necessary in areas where liquid splashes are more likely, for example at cargo manifolds, tank sample points and in the vicinity of compressors or condensers. Skin contact with un-insulated pipes and valves in the cargo area should be avoided.
For fully-pressurised gases, while containment systems will normally be at or near ambient temperature, liquid leaks will quickly flash to their fully-refrigerated temperature. In the event of spillage it is important not to make contact with the spilled material as it can cause chemical and cold burns to the skin and underlying tissues. Where a leak occurs from a pipeline on deck it will usually be dealt with as per the ship or Company’s contingency plan, which will be based on the cargo being carried. The vessel’s response may include measures such as cordoning off the area on deck, applying a spare fire hose directed to spray on the area or activating the deck water spray. In the case of toxic cargoes, operations will normally be resumed when safe conditions can be established.
Appropriate drills help to ensure that the crew are confident in their actions and that the established procedures for handling low temperature cargo are in place and will be followed at all times.
The use of monitoring and alarm systems may also prevent crew members from being unexpectedly exposed to liquefied gases and will provide warnings if areas have become dangerous due to extreme cold.
Symptoms of frostbite
Symptoms of cold burns are very similar to those experienced from burning by high temperatures:
- the skin initially becomes red, but then turns white;
- the affected area is usually painless (before thawing);
- extreme pain in the affected area (after thawing);
- the affected area is hard to the touch;
- confusion or agitation;
- fainting;
- shock.
If the area is left untreated the tissue will die and gangrene may occur.
Treatment of frostbite
The following steps are commonly taken to help to treat frostbite:
- Warm the area rapidly but gently:
- initially using woollen material and/or body heat (eg place frostbitten hands/fingers under the armpit) to ensure heat is applied immediately;
- then place into water with a temperature of 42 °C until affected area has thawed, which may take 15 to 60 minutes;
- if placing the affected area in warm water is not possible, the casualty should be wrapped in warm blankets to allow circulation to re-establish naturally;
- keep the patient in a warm room;
- do not massage the affected area;
- exercise the affected area if possible;
- severe pain may occur on thawing. Give painkillers or morphine if the pain is serious;
- blisters should never be cut, nor clothing removed if it is adhering firmly;
- while warming up, the patient should not be given any alcohol or be allowed to smoke;
- dress the area with sterile dry gauze.
If the affected area does not regain normal colour and sensation, obtain professional medical advice. Note that some hospitals may be unfamiliar with cold burns and frostbite, so a detailed description of the incident may need to accompany the patient to a hospital.
Chemical burns
As shown in Table 4, chemical burns can be caused by ammonia, chlorine, ethylene oxide and propylene oxide. Deck showers and eye baths are provided for water rinsing on gas carriers certified to carry these products. Their locations will usually be dearly indicated so that treatment can be administered as quickly as possible in the event of an accident.
Symptoms
The symptoms are similar to fire burns except that the product may also be absorbed through the skin, causing toxic side effects. Chemical burning is particularly damaging to the eyes. Symptoms include:
- a burning pain with redness of the skin;
- an irritating rash;
- blistering or loss of skin;
- toxic poisoning;
- shock.
Treatment
The following steps are commonly taken to help to treat chemical burns:
- remove patient from source of contamination, including clothing;
- attend first to the eyes and skin:
- wash the eyes thoroughly for ten minutes with large amounts of fresh water;
- wash the skin thoroughly for ten minutes with large amounts of fresh water;
- cover with a sterile dressing.
Seek urgent medical/first-aid attention if the area of the burn is large or the wound involves second or third degree burns.

It is also important to be aware of any toxic effects that may occur following exposure and to seek medical help for these if appropriate. Otherwise, the treatment is as for burns, details of which are contained in the IMO “Medical First Aid Guide (MFAG)” (Reference 1.15).
Other hazards of liquefied gases
As the gas industry moves to processing, such as with floating LNG facilities (FLNGs) and LPG floating production storage and offloading facilities (FPSOs), some of the hazards that exist in upstream processing may now be present onboard marine vessels. Examples of hazards that a ship’s crew may now need to be aware of on such units include trace element impurities such as mercury, and naturally occurring radioactive materials (NORMs) that exist in rock formations at trace levels.
Systems for removal of and/or addressing such hazards will commonly be included as part of the facility processing equipment design, and in operating procedures.
Emergency Planning
The emergency plan
An emergency can occur at any time and in any situation. Effective action may only possible if pre-planned and practical procedures have been developed and are frequently exercised.
When cargo is being transferred, the ship and shore become a combined operational unit and it is during this operation that the greatest overall risk arises. In this respect, the cargo connection is probably the most vulnerable area.
The objective of an emergency plan that covers cargo transfer operations will usually be to make maximum use of the resources of the ship, the terminal and local authority services. The plan will commonly be directed at achieving the following aims, amongst others:
- rescuing and treating casualties;
- safeguarding others;
- minimising damage to property and the environment;
- bringing any incident under control.
Ship emergency procedures
Organisational structure
Effective emergency response requires an emergency organisation, around which detailed procedures may be developed. The international character of ocean shipping and its universally similar command structures lend themselves to the development of a standard approach in ships’ emergency planning. For gas carriers this broad uniformity can be extended further to the development of incident planning. Such standardisation is of advantage since ships’ personnel generally do not continuously serve on the some ship. It is also of advantage in the handling of incidents in port in that terminal emergency planning can be more effective if there is knowledge of the procedures a ship is likely to follow.
Outlined below is a suggested emergency organisational structure for gas carriers in port that has received wide acceptance, although it may not be suited to all situations. The basic structure consists of four elements:
Emergency command centre
In port the emergency command centre will be established in the cargo control room. It will be manned by the senior officer in control of the emergency, supported by another officer and a crew member acting as a messenger. Communication will be maintained with the three other elements, and with the terminal emergency control room, by portable radio or telephone.
Emergency party
The emergency party is a pre-designated group. It is the first team sent to the scene and reports to the emergency command centre on the extent of the incident. The party recommends the action to be taken and the assistance required. The party is under the control of o senior officer and comprises officers and other suitable personnel trained to deal with rescue or fire-fighting .
Bock-up emergency party
The bock-up emergency party stands by to assist the emergency party at the direction of the emergency command centre. The back-up party will be led by an officer and comprises selected personnel.
Engineers group
Some engineering personnel may form port of either emergency party. However, the engineers group is normally under the leadership of the Chief Engineer and has prime responsibility for dealing with an emergency in the main machinery spaces. Additionally, the group provides emergency engineering assistance as directed by the emergency command centre.
Incident plans
In developing plans for dealing with incidents, the following scenarios will commonly be considered:
- checks for missing or trapped personnel;
- collision;
- grounding;
- water leakage into a hold or interbarrier space;
- cargo containment leakage;
- cargo connection rupture, pipeline fracture or cargo spillage;
- lifting of a cargo system relief valve;
- fire in non-cargo areas;
- fire following leakage of cargo;
- loss of cofferdam space heating system;
- fire in a compressor or motor room.
Terminal emergency procedures
Organisational structure
Terminal emergency organisational structure and incident planning are less likely to be standardised than on ships. Terminal plans depend on the size and nature of the terminal and how it is located in relation to other harbour facilities and neighbouring industry.
Whatever the nature and location of a terminal, it will require a fast acting emergency structure under the command of a site incident controller. The incident controller will commonly operate from o designated emergency control room. The organisation will need to be fully responsive at any time of day or night and under shift working conditions.
Read also: Features of cargo delivery LNG/LPG carriers
While frequently responsible for initiation and direction of immediate action in case of a major incident, the emergency organisation at a marine terminal may come under the direction of the port authority. In such cases the port authority may be expected to have a fast acting structure, within its own emergency control centre, that is available at all times. The port authority may also be expected to have means of coordinating assistance from other public services. Generally they will have procedures for issuing warnings to, and evacuation of, the surrounding industry and population. It is common to develop the terminal’s emergency planning, and similar port planning, together and exercise both joinly at suitable intervals.
It is common, when developing procedures, to give guidance to the site incident controller on the scaling of incident severity to provide a check on when to call upon port authority emergency response personnel and services.
Incident plans
In the development of a terminal’s incident plan, the following aspects may be appropriate for consideration, amongst other things:
- Cargo spillage or fire on board a ship alongside the jetty;
- cargo spillage or fire while loading or receiving cargo;
- cargo spillage or fire not associated with loading or receiving cargo.
Removal of Ship from Berth
A gas carrier moored alongside, with a serious incident on board, cannot usually be safely rem d from the berth. Therefore it may be less of a hazard to the port if it is kept alongside, where assistance from shore services can be provided.
In any serious incident associated with cargo transfer, on shore or on a gas carrier, it is usually essential to shut down cargo flow by stopping pumps and closing ESD valves. All gas carriers and all large terminals have a system for the rapid emergency shutdown of cargo transfer (see article “Cargo equipment for gas carriers carrying LNG/LPGEmergency shutdown (ESD) systems“).
In the case of an internal emergency within the terminal, it is often considered good practice to remove gas carriers from the berths in a controlled manner to avoid their possible involvement in the situation.
In all situations, a ship’s removal will most likely be a matter for consultation between the Master, the terminal and the port authority.
Ship to Ship Cargo Transfer
A spillage or fire during ship to ship (STS) cargo transfer operations presents aspects of emergency action that need special consideration. This is one reason why the contingencies and emergency procedures should be fully discussed between the two Masters before operations commence.

An incident on one ship may call for substantial assistance from the emergency resources of the other, to the mutual benefit of both. There may be circumstances, however, when it would be preferable for the ships to separate to minimise the overall risk and, perhaps, allow unobstructed access to the stricken ship by fire tugs and salvage services. These matters are more fully discussed in the STS Guide (Reference 2.14).
Hazards with the Use of Hoses and Marine Loading Arms (MLAs)
For cargo operations of marine terminals, records show that the greatest risk of serious gas escape is concentrated at the ship and shore manifold area. This may be the result of a ship breaking-out from the berth, typically due to strong wind conditions, although strong currents and even ice conditions have caused similar problems. Accidents have also occurred because of human error, lack of training, failure of hoses and malfunction of the MLAs. A further cause is shown to result from poorly maintained transfer equipment.
The main elements of risk can be summarised as follows:
| Dangers to both hoses and MLAs | collision of the berth |
| poor mooring plans and related operating procedures | |
| inadequate mooring line quality | |
| strong wind conditions | |
| interaction between ships alongside and those passing-by | |
| Dangers to hoses | incorrectly specified hoses |
| poorly ma intained hoses | |
| lack of break-away couplings | |
| Dangers to MLAs | lack of on automatic ERS |
| insufficient training for operational personnel | |
| poorly ma intained equipment | |
| the fitting of unsuitable MLAs to small ships | |
| Dangers to pipelines | high surge pressures |
| lack of linked ESD systems | |
| poor pipeline maintenance |
MLAs are the preferred option for the transfer of liquefied gases. Although correct operation and adequate maintenance is required for all transfer systems, hoses require more active management (ie core during handling and operations, and frequent inspections and testing) to achieve an equivalent safety level to MLAs.
A principal danger from a liquefied gas spill is the formation of a vapour cloud. These may be flammable and toxic in nature and can be extensive (see article “Properties of liquefied gasesFlammability/flammable range“). Spill volumes can be effectively limited to small amounts by fitting in-line safety devices on MLAs.
Other dangers include the risk of very cold liquid causing serious frostbite and, possibly, chemical burns (see point “Frosbite” and “Chemical burns” below). In addition, a spillage of a cryogenic liquid may cause metal embrittlement to ship and shore structures. On occasion, this has resulted in ships being removed from service to repair areas of cracked steel.
The primary means of risk reduction at the cargo manifold include:
- the provision of a safe berth, clear from other manoeuvring ships;
- a well designed mooring system (with appropriate operating procedures);
- properly specified cargo handling equipment;
- means of spill limitation built into the transfer equipment (drip tray and the use of a water curtain on LNG carriers);
- the provision of a linked ship/shore ESD;
- methods of surge pressure alleviation;
- restricted areas around ship and shore manifolds;
- well organised handling, maintenance and testing procedures;
- good training – particularly for operations personnel.

