Latent Heat – Definition and Pronunciation
What is Latent Heat?
Latent heat is the heat energy required to cause a change of state of a substance from solid to liquid (or vice versa – and called the latent heat of fusion), or from liquid to vapour (or vice versa – and cal led the latent heat of vaporisation). These phase changes occur without change of temperature at the melting point and the boiling point.
Examples of Latent Heat
Because of lack of data for polystyrene foam we used the latent heat of fusion for styrene monomer (105 kJ/kg) along with the density of polystyrene foam (26,5 kg/m3) to estimate a melting rate of about 3 centimetres per minute.
From Time Based Heat Transfer“How does time based heat transfer on a tanker (LNG)?”.
The calculations used to determine the tabulated cool-down quantities consider the overall tank surface area, the overall heat capacity of the cargo containment system for a stabilised temperature gradient across the tank insulation and the latent heat of the vaporised liquefied gas.
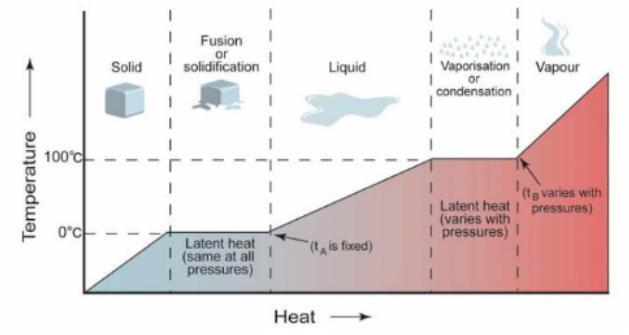
A – rise in temperature as ice absorbs heat;
B – absorption of latent heat of fusion;
C – rise in temperature as liquid water absorbs heat;
D – water boils and absorbs latent heat of vaporisation;
E – steam absorbs heat and thus increases its temperature (from Liquefied Natural Gas Fundamental Knowledge and Understanding“Chemical and physical properties of LNG, fundamentals”)
It is important to note that the saturation temperature is not the only parameter affected by the evaporating pressure conditions. The value for the latent heat of vaporisation also varies significantly, depending on evaporation pressure.
From Properties of liquefied gases“Liquefied natural gas properties and conditions of its transportation”.
